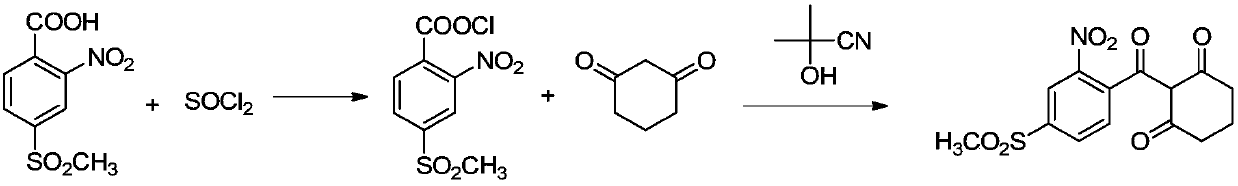
Refining method of 2-nitro-4-methanesulfonylbenzoic acid
A technology of methanesulfonyl benzoic acid and purification method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc. The problem of low purity of sulcotrione original drug, to achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In a 500ml reaction flask, add 0.8g of sodium hydroxide and 158g of anhydrous methanol, stir at room temperature for 30 minutes, so that all the sodium hydroxide is dissolved in methanol; add 2-nitro-4-methylsulfonylbenzoic acid Crude product 50g (95% by weight), stirred, heated to 65°C and refluxed for 30 minutes, cooled to 60°C, filtered at 60°C, cooled the filtrate to 5°C, filtered at 5°C, dried to obtain 2-nitro- 45.8 g of 4-methylsulfonylbenzoic acid, the content was 98.6% by weight, and the yield was 95.07%.
Embodiment 2
[0042] In a 500ml reaction flask, add 1g of potassium hydroxide and 160g of absolute ethanol, and stir at room temperature for 30 minutes, so that all of the potassium hydroxide is dissolved in ethanol; add the crude product of 2-nitro-4-methylsulfonylbenzoic acid 50g (95% by weight), stir, heat up to 67°C and reflux for 30 minutes, cool to 60°C, filter at 60°C, cool the filtrate to 5°C, filter at 5°C, and dry to obtain 2-nitro-4 - 45.7 g of methylsulfonylbenzoic acid, the content is 98.8% by weight, and the yield is 95.05%.
Embodiment 3-7、 comparative example 1
[0044]Purify 2-nitro-4-methanesulfonylbenzoic acid according to the method of Example 1, the difference is that the amount of sodium hydroxide added is as shown in Table 1. The weight, content and yield of 2-nitro-4-methylsulfonylbenzoic acid finally obtained are shown in Table 1.
[0045] Table 1
[0046] Sodium hydroxide (g) Weight (g) Content (weight%) Yield (%) Example 1 0.8 45.8 98.6 95.07 Example 3 0.7 45.0 98.7 94.13 Example 4 0.9 45.4 98.6 94.24 Example 5 0.6 44.5 98.3 92.47 Example 6 2 38.0 98.7 78.96 Example 7 3 33.5 97.9 69.05 Comparative example 1 0 40 99.5 83.79
[0047] As can be seen from the above Table 1, by making the amount of base 0.09-0.12 mol relative to 1 mol of the 2-nitro-4-methylsulfonylbenzoic acid, the 2-nitro-4-methanol obtained by refining can be further increased. Sulfonylbenzoic acid weight and its content, yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com