Salidroside derivative and preparation method and application thereof
A compound and stereoisomer technology, applied in the field of salidroside derivatives and their preparation, can solve the problems of fast metabolism and low bioavailability, achieve broad application prospects, overcome low bioavailability, and excellent neuroprotection effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
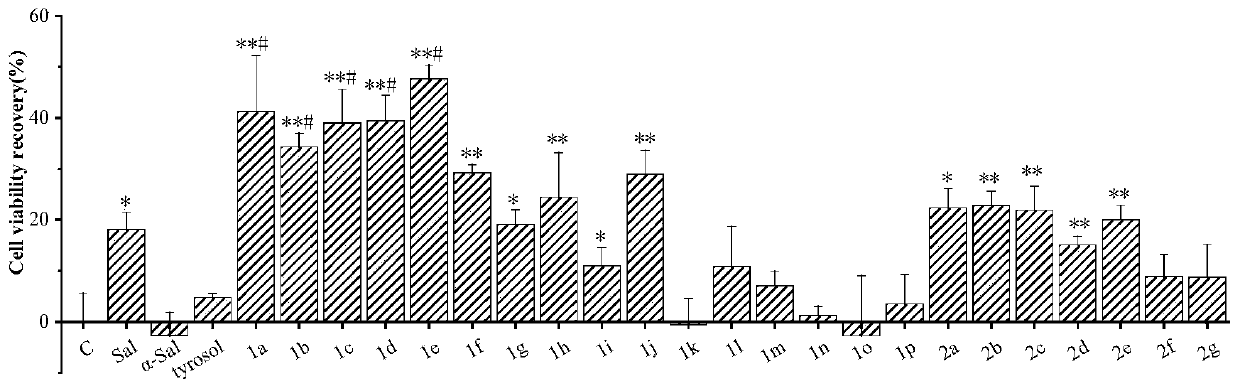
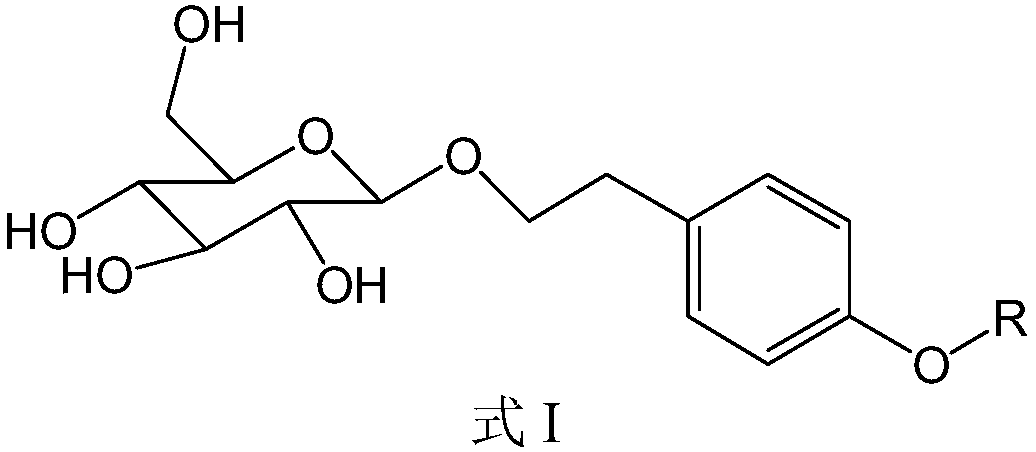
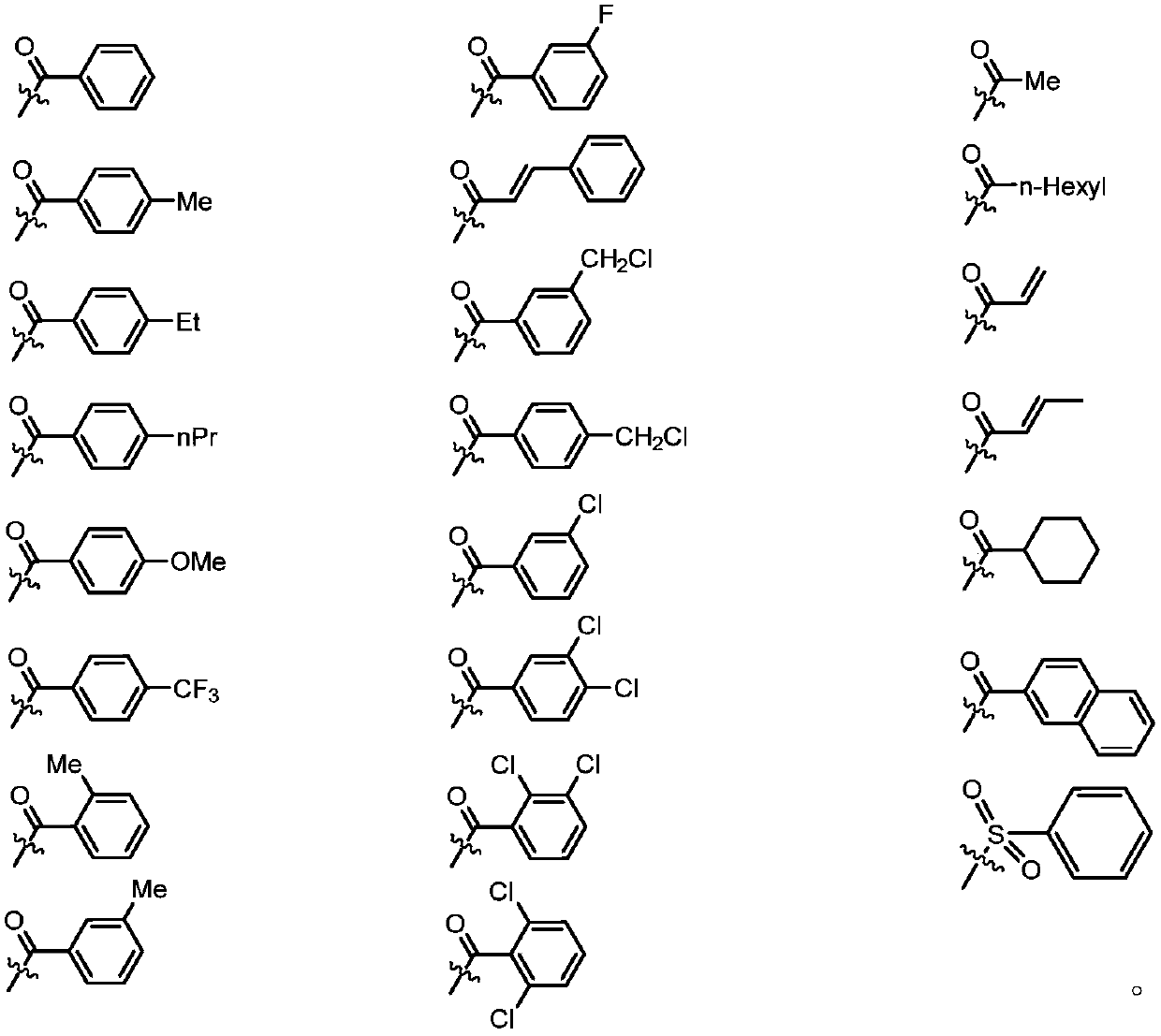
[0040] Embodiment 1, the preparation of salidroside derivative 1a of the present invention
[0041]
[0042] Add 3g (0.01mol) of salidroside sal, 1.06g (0.01mol) of sodium carbonate, 0.05g (0.155mmol) of tetrabutylammonium bromide, and 10ml of water into a 50ml three-necked flask. In an ice bath, stir until the solid is completely dissolved and the temperature of the system drops to -5°C to 5°C. Dissolve 0.01mol benzoyl chloride in 10ml ethyl acetate and add to the system. TLC (CHCl 3 :CH 3OH=4:1) monitor the reaction, and stop the reaction when the raw material point disappears. Ethyl acetate was added to the system after the reaction was completed, liquid separation was carried out, and extraction was performed 3 times, each time using 10 ml of ethyl acetate. The organic phases were combined, dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a white solid. With silica gel as the stationary phase, (CHCl 3 -CH 3 OH) ...
Embodiment 2
[0043] Embodiment 2, the preparation of salidroside derivative 1b of the present invention
[0044]
[0045] With reference to the method of Example 1, benzoyl chloride was replaced by p-toluoyl chloride to obtain salidroside derivative 1b of the present invention: p-(p-toluyloxy)phenethyl-β-D -Glucopyranoside 2.4g, light yellow solid, yield 55%, the purity is 96% by peak area detected by HPLC, the molecular weight is determined by MS, and the structure is confirmed by combining NMR carbon spectrum and hydrogen spectrum. 1 HNMR(400MHz,MeOD)δ8.05(d,J=8.2Hz,2H),7.37(s,2H),7.35(t,J=2.0Hz,2H),7.21–7.03(m,2H),4.41– 4.28(m,1H),4.21–4.06(m,1H),3.90(dd,J=12.0,1.4Hz,1H),3.81(dt,J=9.7,7.2Hz,1H),3.75–3.66(m, 1H), 3.39(dd, J=12.8, 5.1Hz, 1H), 3.33(ddd, J=7.5, 5.7, 2.7Hz, 2H), 3.26–3.20(m, 1H), 3.07–2.88(m, 2H) ,2.43(d,J=17.2Hz,3H). 13 C NMR (101MHz, MeOD) δ165.47, 149.46, 144.66, 136.59, 129.73, 129.44, 129.09, 126.64, 121.24, 103.00, 76.72, 76.57, 73.72, 70.26, 70.14, 61.40, 35.30....
Embodiment 3
[0046] Embodiment 3, the preparation of salidroside derivative 1c of the present invention
[0047]
[0048] With reference to the method of Example 1, benzoyl chloride was replaced by p-ethylbenzoyl chloride to obtain salidroside derivative 1c of the present invention: p-(p-ethylbenzoyloxy)phenethyl-β-D -Glucopyranoside 0.9g, white solid, yield 42%, the purity was detected by HPLC, and the peak area was 95%, the molecular weight was determined by MS, and the structure was confirmed by combining NMR carbon spectrum and hydrogen spectrum. 1 HNMR(500MHz,MeOD)δ8.12–8.05(m,2H),7.39(d,J=8.4Hz,2H),7.36(d,J=8.5Hz,2H),7.16–7.09(m,2H), 4.35(d,J=7.8Hz,1H),4.19–4.10(m,1H),3.89(dt,J=9.5,4.9Hz,1H),3.81(dt,J=9.7,7.2Hz,1H),3.75 –3.66(m,1H),3.43–3.35(m,1H),3.33(ddd,J=7.9,6.4,4.5Hz,2H),3.23(dd,J=9.1,7.8Hz,1H),3.03–2.95 (m,2H),2.79–2.71(m,2H),1.29(t,J=7.6Hz,3H). 13 C NMR (126MHz, MeOD) δ165.47, 150.83, 149.48, 136.59, 129.86, 129.73, 127.93, 126.88, 121.24, 103.01, 76.73, 76.58, 73.73, 70...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com