High-neutralizing-activity anti-SARS-CoV-2 fully-humanized monoclonal antibody and application thereof
A monoclonal antibody, fully human technology, applied in the field of microbiology and immunology, can solve the problems of difficult large-scale preparation, batch differences, limited plasma donation sources, etc., achieve low immunogenicity, good stability, good The effect of neutralizing the protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Screening and Preparation of Human Anti-SARS-CoV-2 Monoclonal Antibody
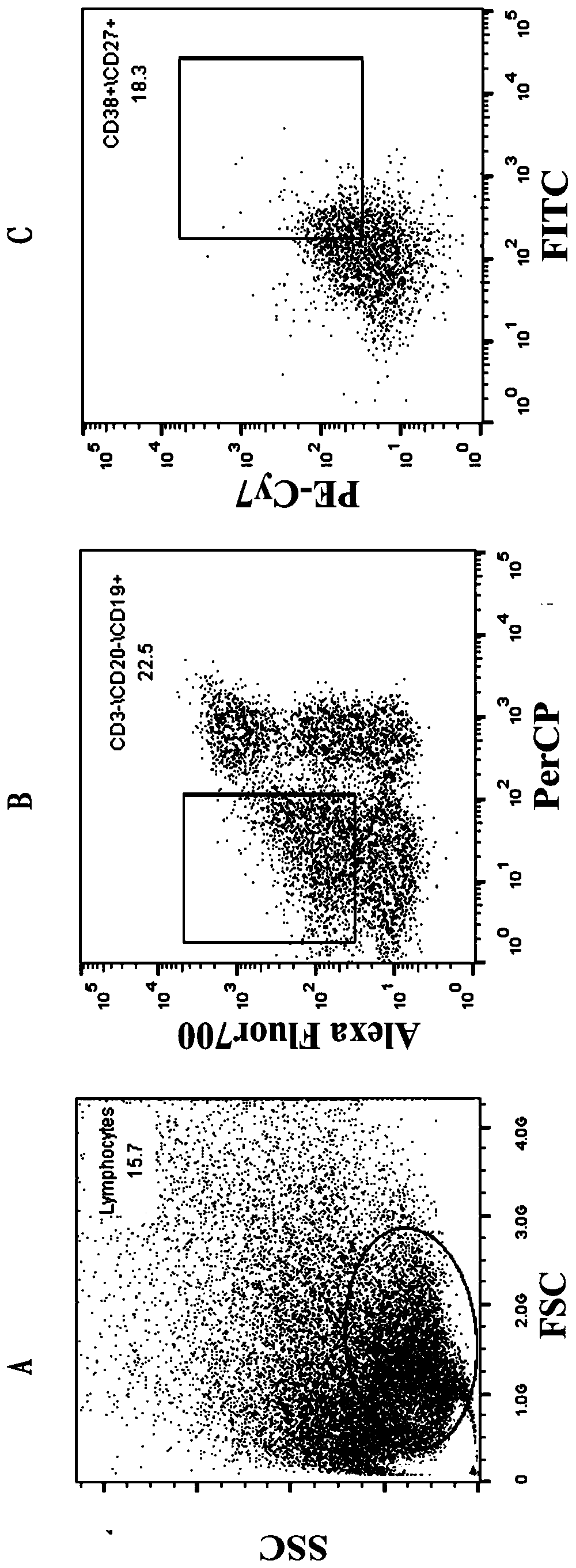
[0032] 1.1 Sorting single cells by flow cytometry
[0033] The collected blood samples were separated from PBMC by Ficoll density gradient centrifugation, and the process was as follows:
[0034] 1.1.1 Take fresh anticoagulated whole blood (EDTA anticoagulated) and dilute the whole blood with an equal volume of PBS.
[0035] 1.1.2 Add a certain volume of separation liquid into the centrifuge tube, spread the diluted blood sample above the liquid surface of the separation liquid, and keep the interface between the two liquid surfaces clear. The volume of separation medium, anticoagulated undiluted whole blood, and PBS (or normal saline) is 1:1:1.
[0036] 1.1.3 Trim, room temperature, horizontal rotor 700-800g (2000-2500rpm), acceleration 3-4 acc, centrifuge for 20-30min.
[0037] 1.1.4 After centrifugation, the bottom of the tube is red blood cells, the middle layer is the separation l...
Embodiment 2
[0091] Example 2 Screening of binding activity of human anti-SARS-CoV-2 monoclonal antibody to S protein and analysis of antibody 4A8 recognition epitope
[0092] 2.1 Coating: Dilute the recombinant S antigen, S1 antigen, RBD antigen and S2 antigen with the coating solution to a concentration of 2 μg / mL, coat the microtiter plate, 100 μL per well, and coat overnight at 4 °C.
[0093] 2.2 Blocking: add 300 μL of PBST washing solution to each well, wash 3 times × 3 min each time; tap the liquid in the well, add 2% BSA, 200 μL / well, block at 37°C for 1 hour.
[0094] 2.3 Sample incubation: Add 300 μL PBST washing solution to each well, wash 3 times × 3min / time; pat the liquid in the well, add purified monoclonal antibody diluted in PBS, the first well 9ug / ml, 3-fold serial dilution 100 μL / well, 37 Incubate at ℃ for 1h.
[0095] 2.4 Secondary antibody incubation: wash, same as above; add HRP goat anti-human F C Secondary antibody (diluted 1:20000), 100 μL / well, incubated at 37°C...
Embodiment 3
[0102] Neutralizing activity analysis on cell model during embodiment 3 SARS-CoV-2 infection
[0103] 3.1 After digesting Vero E6 cells with 0.25% trypsin, dilute to 3×10 with medium (DMEM+10% FBS) 5 The concentration of cells / mL was inoculated into 96-well cell culture plates with an inoculation volume of 100 μL / well, and cultured overnight in a 5% CO2 cell incubator at 37°C.
[0104] 3.2 On the day of the experiment, add the purified monoclonal antibody to the 96-well culture plate from the initial concentration (4A8 monoclonal antibody initial concentration 154ug / ml, 3-fold serial dilution, volume 120μL / well; then add 120μL per well COVID-19 virus suspension (dilute virus with DMEM+2%FBS, add 100TCLD 50 / well), mix thoroughly, and incubate for 1 h in a cell culture incubator.
[0105] 3.3 Discard the cell culture supernatant in the 96-well plate, and add 200 μL of co-incubated virus-antibody mixed suspension to each well; set up a survival control (no virus and antibody) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Equilibrium dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com