A fully human monoclonal antibody with high neutralizing activity against chikungunya and its application
A technology of monoclonal antibody and chikungunya virus, which is applied in the field of microbiology and immunology, can solve the problems of non-specific therapeutic drugs, achieve low immunogenicity, good stability, and improve the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
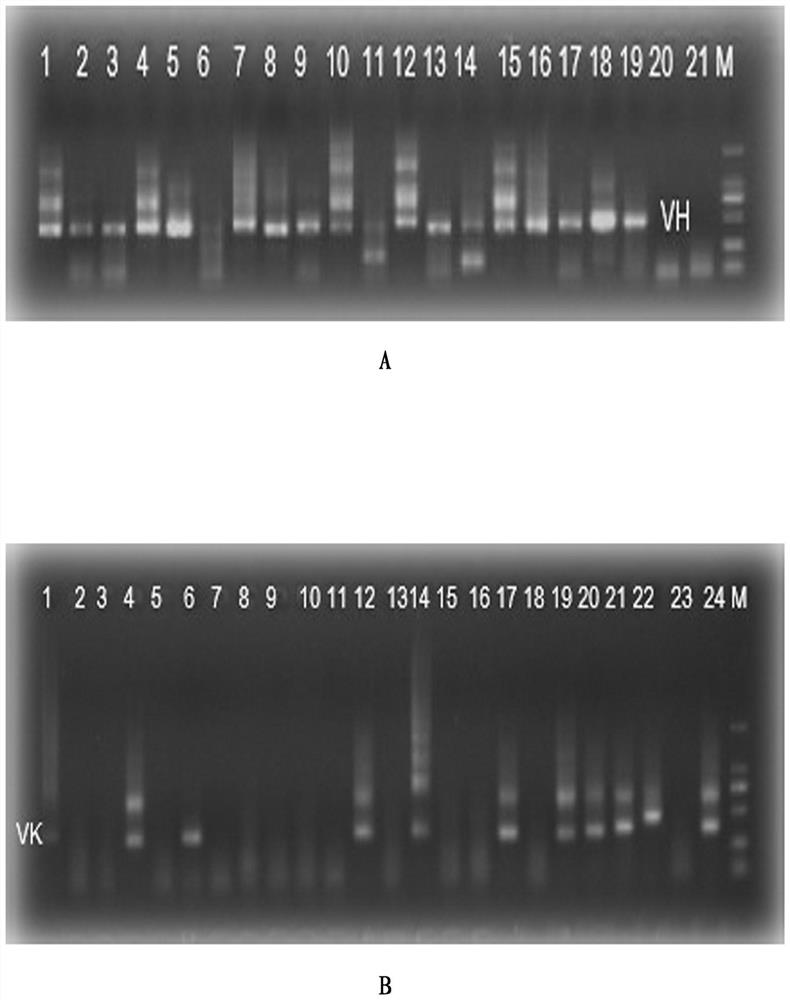
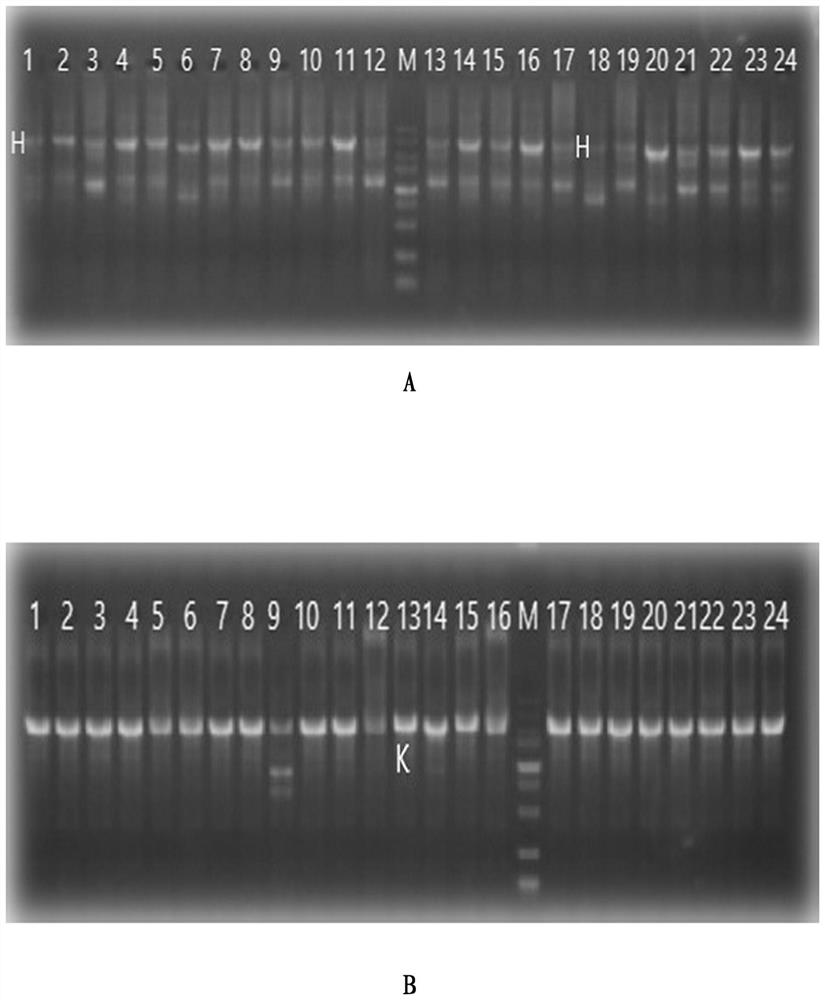
Embodiment 1
[0029] Example 1. Screening and Preparation of Human Anti-Chikungunya Virus Monoclonal Antibody
[0030] 1.1 Blood sample collection
[0031] After obtaining informed consent, Guangzhou Eighth People's Hospital provided peripheral blood from a chikungunya infected patient 15 days after recovery for subsequent experiments.
[0032] 1.2 Sorting single cells by flow cytometry
[0033] The collected blood samples were separated from PBMC by Ficoll density gradient centrifugation, and the process was as follows:
[0034] 1.2.1 Take fresh anticoagulated whole blood (EDTA anticoagulated) and dilute the whole blood with an equal volume of PBS.
[0035] 1.2.2 Add a certain volume of separation liquid into the centrifuge tube, spread the diluted blood sample above the liquid surface of the separation liquid, and keep the interface between the two liquid surfaces clear. The volume of separation medium, anticoagulated undiluted whole blood, and PBS (or normal saline) is 1:1:1.
[0036...
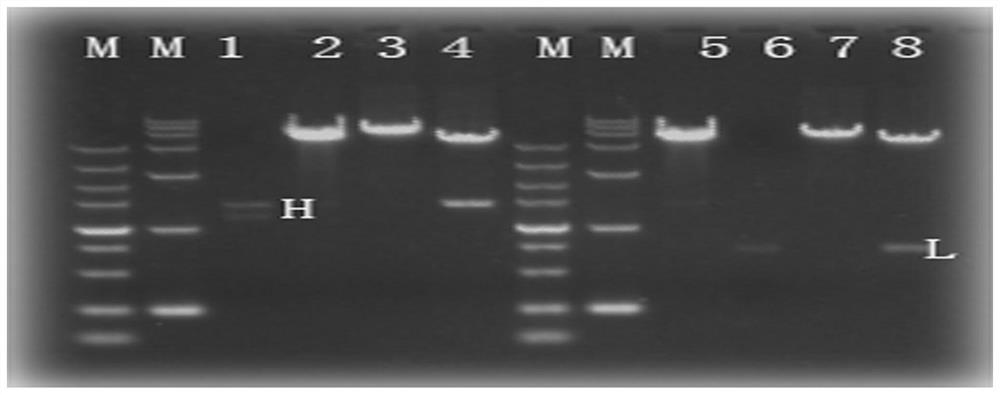
Embodiment 2
[0118] Example 2. Cell neutralization experiment
[0119] 2.1 The day before the experiment, the Vero cells were diluted to 1.5×10 with medium (MEM+10% FBS) 5 Cells / mL concentration, inoculated into 96-well cell culture plate, inoculation volume 200 μL / well, cultured in 37°C 5% CO2 cell incubator;
[0120] 2.2 On the day of the experiment, the purified monoclonal antibody was mixed with medium MEM+2% FBS from the initial concentration (8D1 monoclonal antibody initial concentration 100ug / ml, Ab2 control antibody 200ug / ml (refer to the 8B10F8 heavy chain and light chain variable Region sequence, chemically synthesized variable region gene and obtained full-length antibody gene by fusion PCR technology, cloned into the expression vector pMH vector, transfected CHO-S cells, obtained and preserved by Protein A affinity chromatography purification, named by the laboratory Ab2), 4-fold dilution, added to a 96-well culture plate with a volume of 120 μL / well; then 120 μL of chikunguny...
Embodiment 3
[0125] Example 3. Research on monoclonal antibody recognition epitope
[0126] 3.1 ELISA screening for specific clones: Coat anti-Chikungunya virus monoclonal antibody (100 μg / mL, 150 μL / well) on a 96-well enzyme-linked plate, overnight at 4°C; discard the coating liquid, add 5 mg / mL BSA to block Buffer (0.1mol / L NaHCO3, pH8.6) was blocked at 4°C for 2h; the liquid was poured off, washed 6 times with TBST (1mL / L Tween-20, TBS); 100μL of the phage loop 7 peptide library was added (the original library used TBST According to 1:10 dilution, containing about phage 2×10 11 pfu / 100μL), incubated at room temperature for 30min with gentle shaking; discarded liquid, washed 10 times with TBST to wash off unbound phage; added 100μL eluent (1mg / mL BSA, 0.2mol / L Glycine-HCl, pH2.2 ), shake gently at room temperature for 10 minutes, quickly suck out the liquid and add 15 μL of neutralizing solution (1mol / L Tris-HCl, pH9.1) to neutralize; take 1 μL of the eluted phage to measure the titer, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com