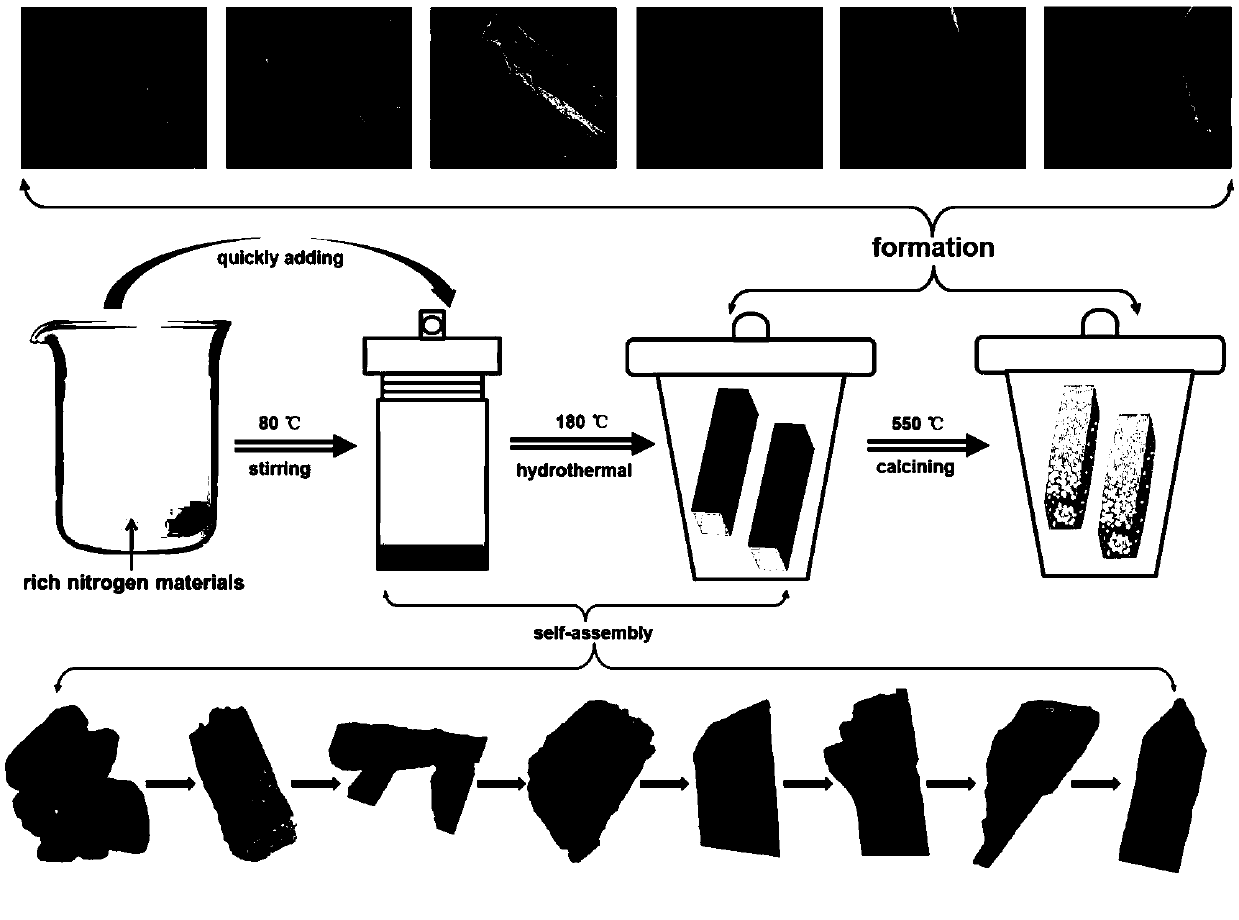
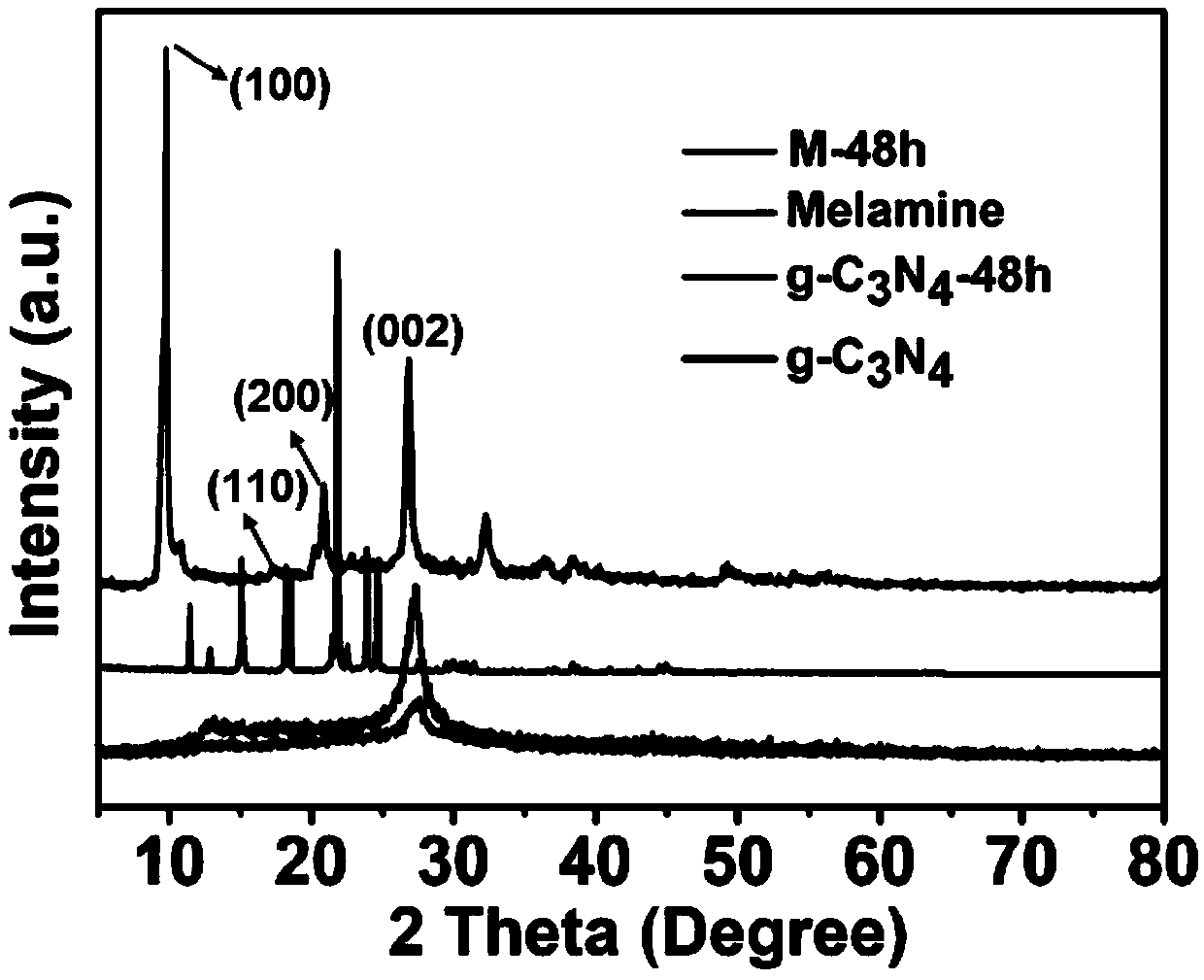
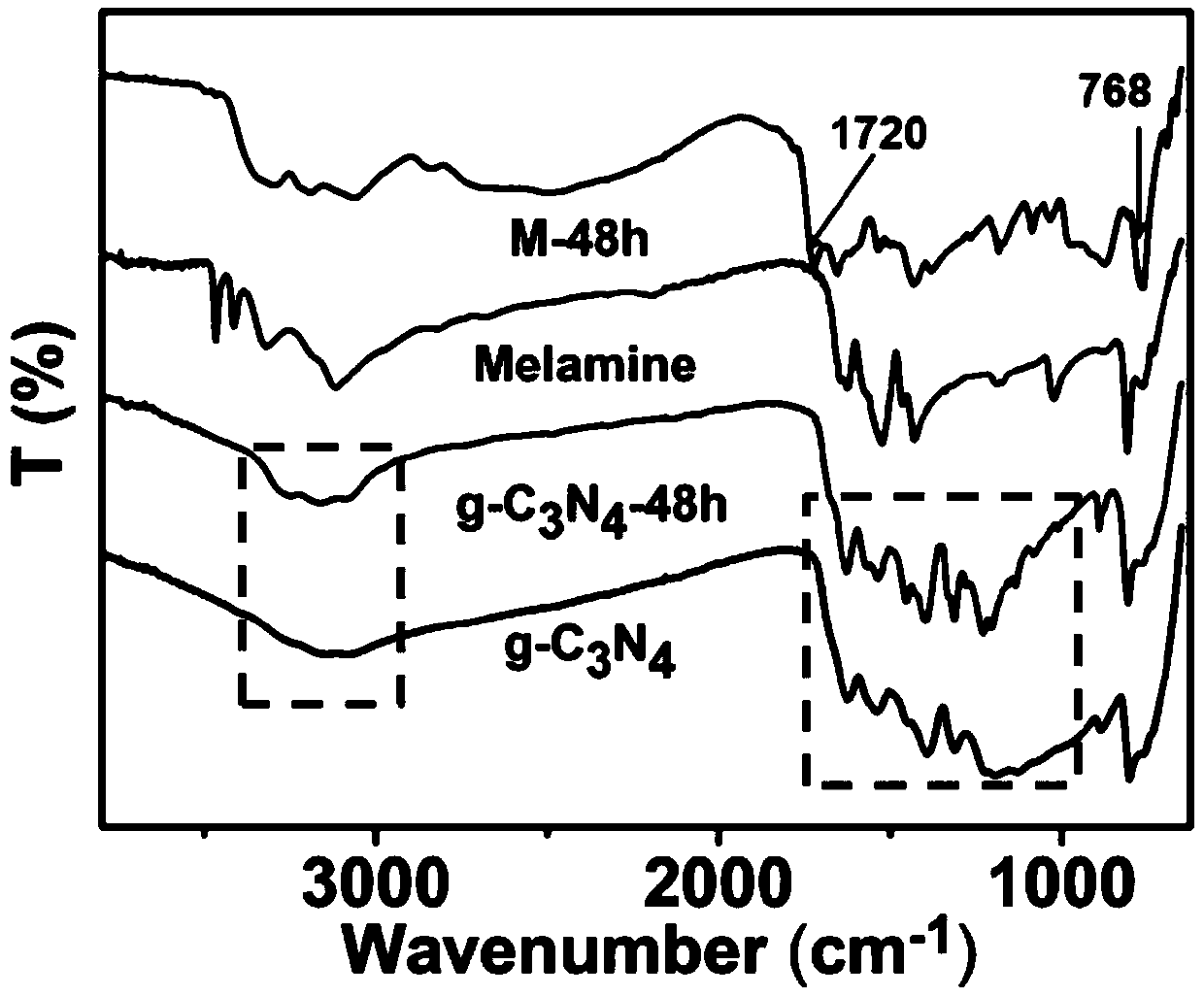
Preparation method of hollow porous prismatic graphite phase carbon nitride
A graphitic carbon nitride, prismatic technology, applied in chemical instruments and methods, catalyst activation/preparation, hydrogen production, etc., can solve the problems of lack of universality of supramolecular precursors and inability to maintain morphology, etc. To achieve the effect of being conducive to separation and migration, improving catalytic performance, and increasing specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Implementation Example 1: g-C 3 N 4 -12h preparation and photocatalytic performance test
[0037] In the first step, 2 g of melamine was dissolved in 80 mL of deionized water, heated and stirred at 80 °C for 120 min;
[0038] In the second step, pour the reaction solution of the first step into a 200 mL reactor while it is hot, and after 12 h of hydrothermal reaction at 180 °C, cool to room temperature;
[0039] In the third step, after washing the solid obtained in the second step reaction several times, drying at 60° C. to obtain the prismatic supramolecular precursor M-12 h;
[0040] In the fourth step, the prismatic supramolecular precursor obtained in the third step was calcined at 550±10 °C for 8 h to prepare a hollow porous prismatic graphitic carbon nitride g-C 3 N 4 -12h.
[0041] Step 5, weigh 20 mg g-C 3 N 4 -12h In the photocatalytic reaction tank, add 90 mL deionized water, 10 mL triethanolamine and 3 wt.% Pt to produce hydrogen under visible light. ...
Embodiment 2
[0043] Implementation example 2: g-C 3 N 4 -18h preparation and photocatalytic performance test
[0044] In the first step, 2 g of melamine was dissolved in 80 mL of deionized water, heated and stirred at 80 °C for 120 min;
[0045] In the second step, pour the reaction solution of the first step into a 200 mL reaction kettle while it is hot, and after a hydrothermal reaction at 180 °C for 18 h, cool to room temperature;
[0046] In the third step, the solid obtained in the second step reaction is washed several times, and then dried at 60° C. to obtain the prismatic supramolecular precursor M-18 h;
[0047] In the fourth step, the prismatic supramolecular precursor obtained in the third step was calcined at 550±10 °C for 8 h to prepare a hollow porous prismatic graphitic carbon nitride g-C 3 N 4 -18h.
[0048] Step 5, weigh 20 mg g-C 3 N 4 -18h In the photocatalytic reaction tank, add 90 mL deionized water, 10 mL triethanolamine and 3 wt.% Pt to produce hydrogen under ...
Embodiment 3
[0050] Implementation Example 3: g-C 3 N 4 Preparation of -21h and photocatalytic performance test
[0051] In the first step, 2 g of melamine was dissolved in 80 mL of deionized water, heated and stirred at 80 °C for 120 min;
[0052] In the second step, pour the reaction solution of the first step into a 200 mL reactor while it is hot, and after 180 °C hydrothermal reaction for 21 h, cool to room temperature;
[0053] In the third step, the solid obtained in the second step reaction was washed several times, and then dried at 60°C to obtain the prismatic supramolecular precursor M-21 h;
[0054] In the fourth step, the prismatic supramolecular precursor obtained in the third step was calcined at 550±10 °C for 8 h to prepare a hollow porous prismatic graphitic carbon nitride g-C 3 N 4 -21h.
[0055] Step 5, weigh 20 mg g-C 3 N 4 -18h In the photocatalytic reaction tank, add 90 mL deionized water, 10 mL triethanolamine and 3 wt.% Pt to produce hydrogen under visible lig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com