Fluorescent sensing material, preparation method thereof and application thereof in high-sensitivity detection of explosives
A technology for fluorescent materials and explosives, applied in the field of organic fluorescent sensing materials, can solve problems such as easy photooxidation, low luminous efficiency, and poor photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
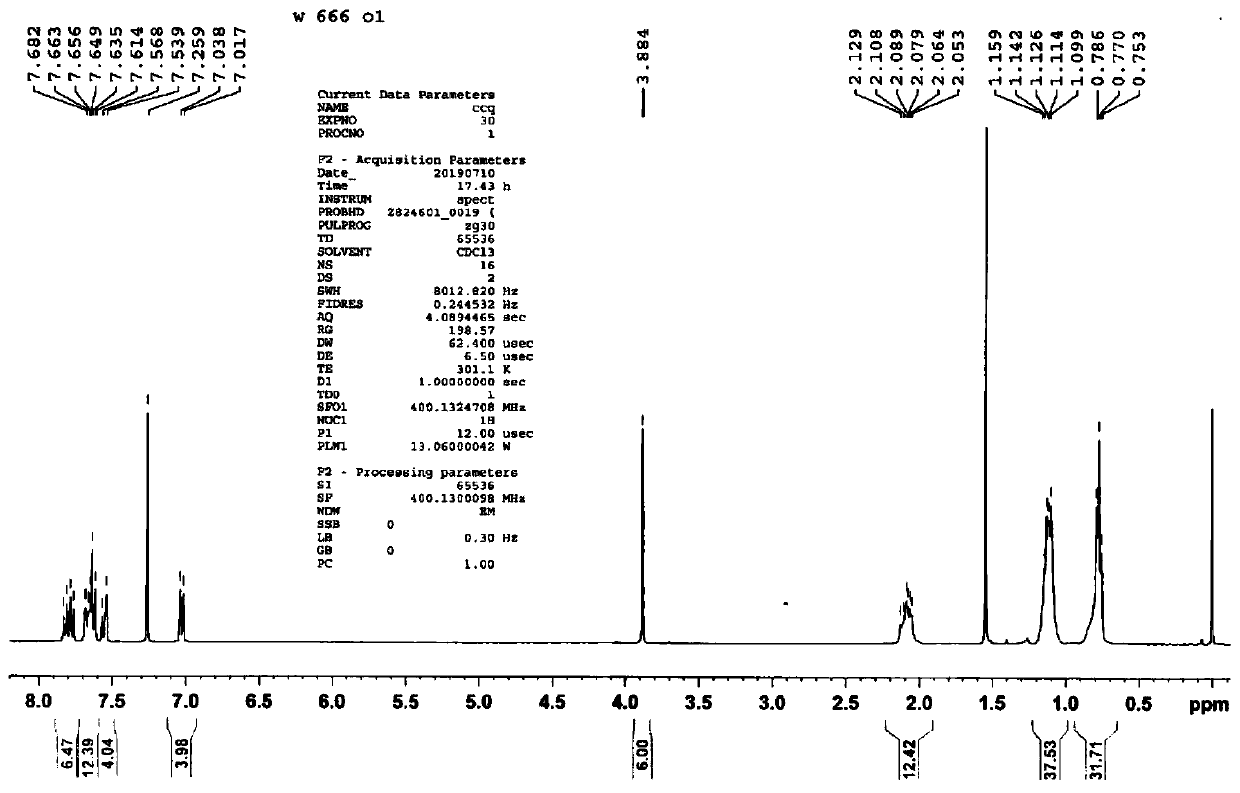
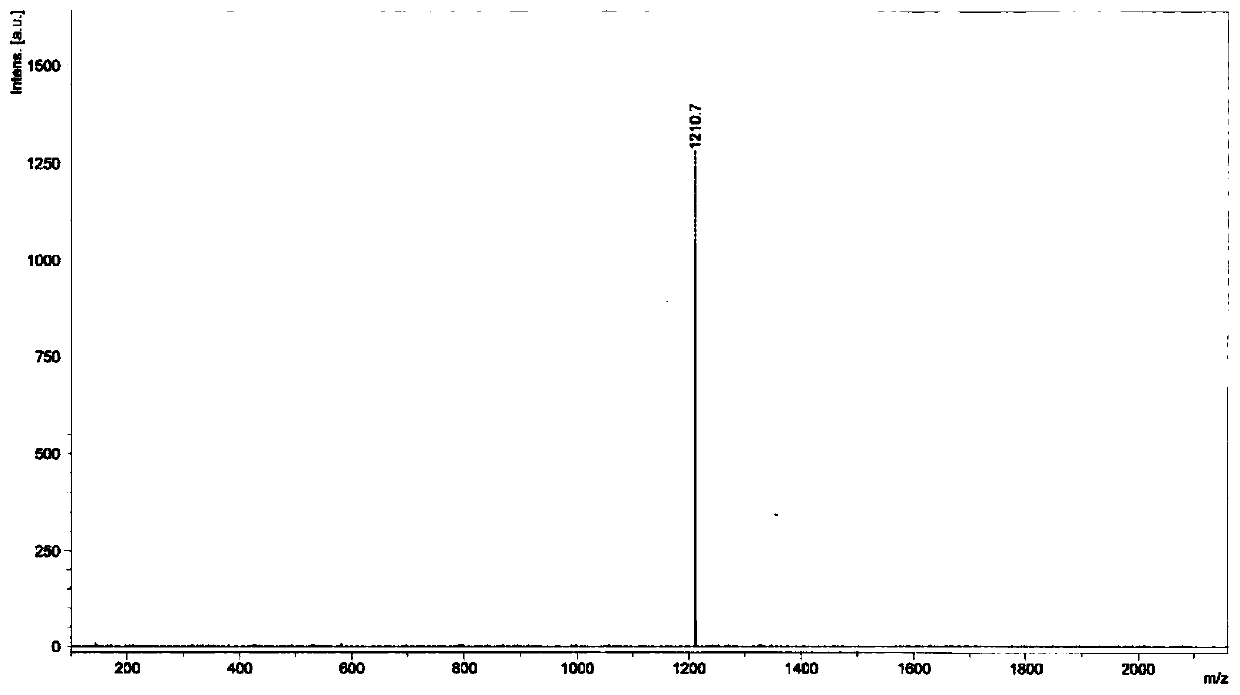
Embodiment 1
[0108] Preparation of compound A, the preparation method is as follows:
[0109]
[0110] (1) Add 3 g of 4-bromoanisole, 4.5 g of bis-valeryl diboron, 4.9 g of potassium acetate, and 0.5 g of 1,1'-bis(diphenylphosphino)ferrocene into a 100 mL round bottom flask Palladium(II) dichloride was added with 20 ml of anhydrous 1,4-dioxane, argon gas was introduced to exhaust oxygen, and the reaction was carried out at 80 degrees Celsius for 8 hours, and the obtained product was obtained after separation by column chromatography.
[0111] (2) Take 2 grams of the product obtained in step (1), add 20 mL of 1,4-dioxane and 4 mL of water into the mixture, and add 4 grams of 9,9-dihexyl-2,7-dibromofluorene , 3.3 grams of potassium carbonate, 0.5 grams of tetrakis (triphenylphosphine) palladium, passed through argon to exhaust oxygen, and reacted for 8 hours at 80 degrees Celsius, and the resulting product was obtained after separation by column chromatography.
[0112] (3) Add 2 grams o...
Embodiment 2
[0121] Preparation of compound B, the preparation method is as follows:
[0122]
[0123] (1) Add 2 grams of 4-bromophenol, 1 gram of 2-butanol, 30 milliliters of tetrahydrofuran, and 3 grams of triphenylphosphine into a round-bottomed flask, pass through argon to exhaust oxygen, and then slowly add 2.8 1 g of diisopropyl azodicarboxylate, returned to normal temperature after the dropwise addition, stirred for 5 hours, and obtained product was obtained after separation by column chromatography.
[0124] (2) Add 1.5 grams of the product obtained in step (1) to a 100mL round bottom flask, add 2 grams of bisvaleryl diboron, 2 grams of potassium acetate and 0.3 grams of 1,1'-bis(diphenylphosphino) ) Ferrocene palladium dichloride (II) was added with 30 milliliters of anhydrous 1,4-dioxane, argon was passed through to exhaust oxygen, and reacted at 80 degrees Celsius for 8 hours, and the obtained product was obtained after separation by column chromatography.
[0125] (3) Add 1...
Embodiment 3
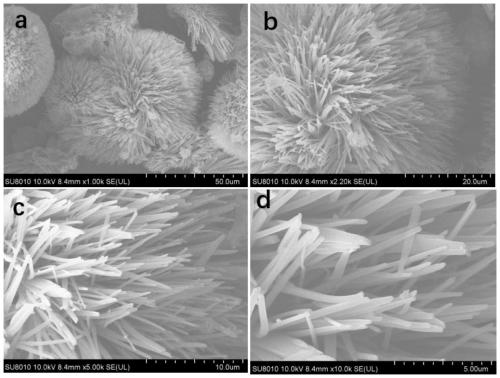
[0137] Compound A in Example 1 and Compound B in Example 2 are dissolved in a good solvent, and then a poor solvent is added, the good solvent is chloroform, the poor solvent is methanol, and the volume ratio of the good solvent to the poor solvent is 1: 15. After standing still, a suspension of organic semiconductor microspheres with different fluorescent responses to several types of explosives was obtained by self-assembly. SEM images such as Figure 11 shown. Such as Figure 12 As shown, the fluorescence intensity of the fluorescent sensing material self-assembled by Compound A decreased by 70% after 4 hours of continuous illumination, while the fluorescence intensity of the organic fluorescent sensing material co-assembled by the two molecules only decreased after 4 hours of continuous illumination. 12%, indicating that after adding benzothiadiazole derivatives for co-assembly, the photostability is greatly improved. Another small amount of aggregates was placed in an ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com