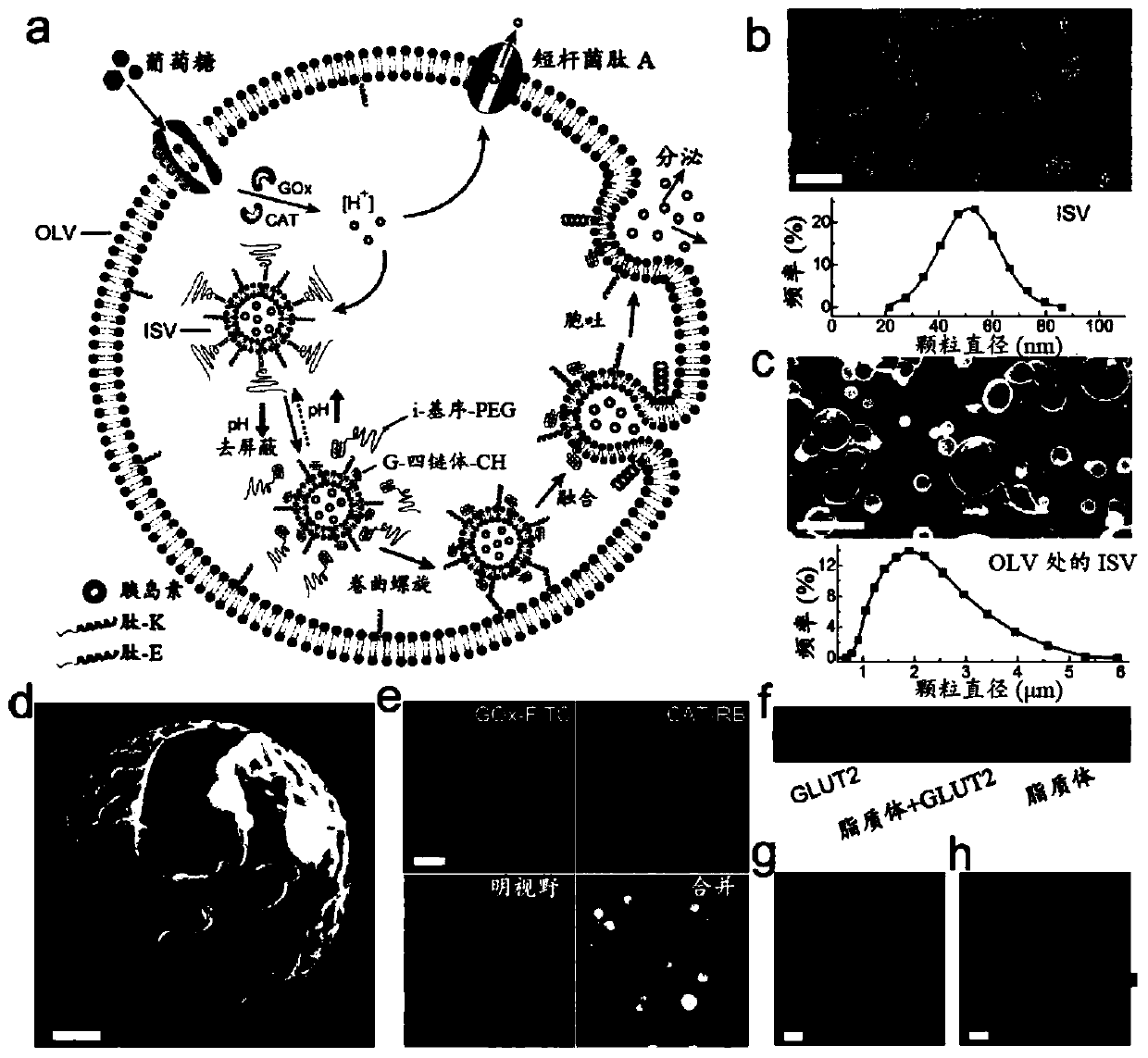
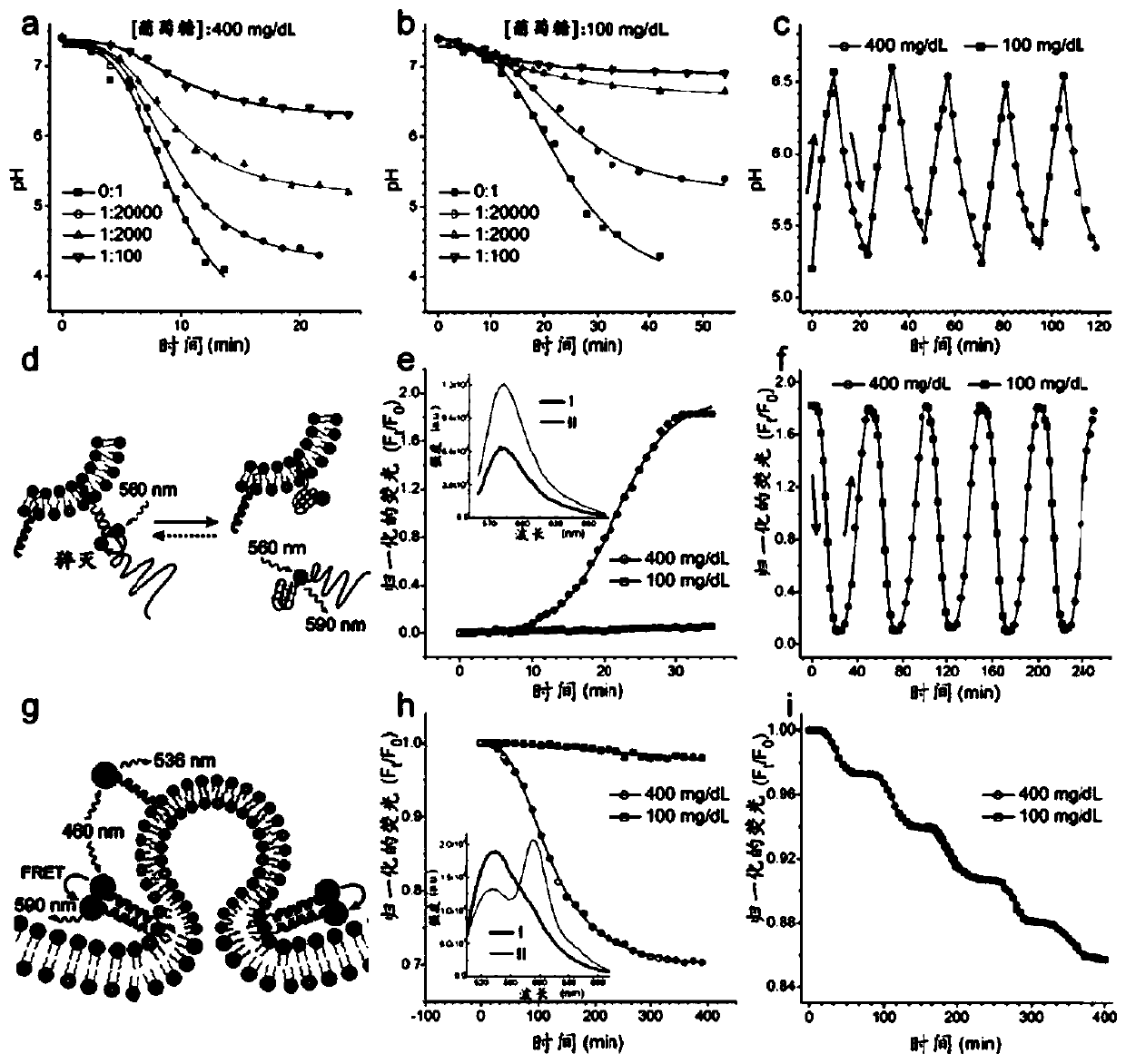
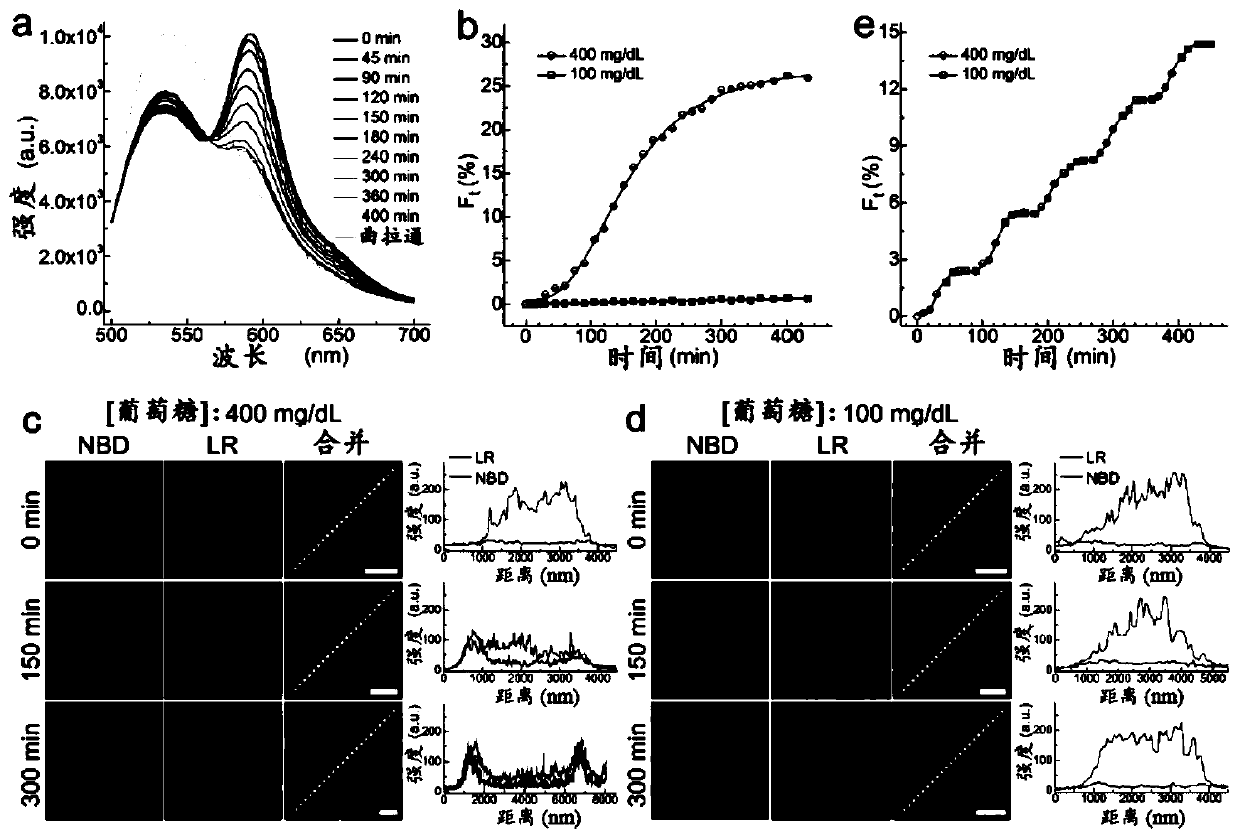
ARTIFICIAL beta-CELLS AND METHODS OF USE THEREOF
A technology of granule and membrane fusion, applied in the field of treatment of diseases, can solve the problems of elusive replication and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0135] Materials and general methods.
[0136] Material. All lipids, including egg phosphatidylcholine (EPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), dipalmitoylphosphatidylcholine (DPPC), 1,2- Dioleoyl-sn glycerol-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (ammonium salt) and 1,2-dioleoyl-sn - Glycerol-3-Phosphatidylethanolamine - Lissamine - Rhodamine B, all purchased from Avanti Polar Lipids, Inc. All peptides were synthesized by Biochem Co., Ltd. (Shanghai, China). All DNA was purchased from DNA Technologies, Inc. (Coralville, IA, USA). Cholesterol, glucose oxidase, catalase, gramicidin A, methoxypolyethylene glycol maleimide (mPEG-Mal, molecular weight = 5000), phenylmethylsulfonyl fluoride (PMSF), Dialkyl β-D-maltoside (DDM) and 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) were purchased from Sigma-Aldrich. Human recombinant insulin was purchased from Life Technology. Anti-Glucose Transporter 2 antibody was purchased fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com