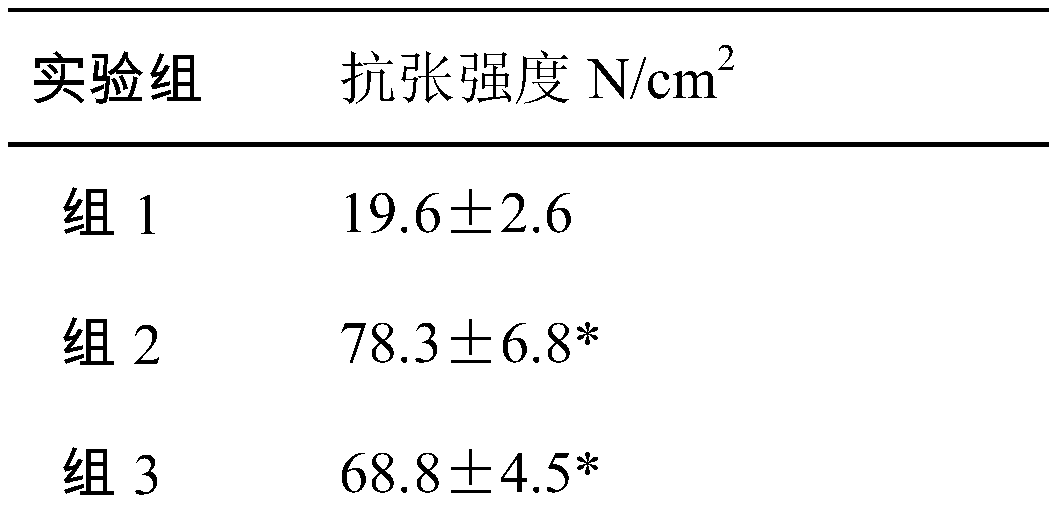
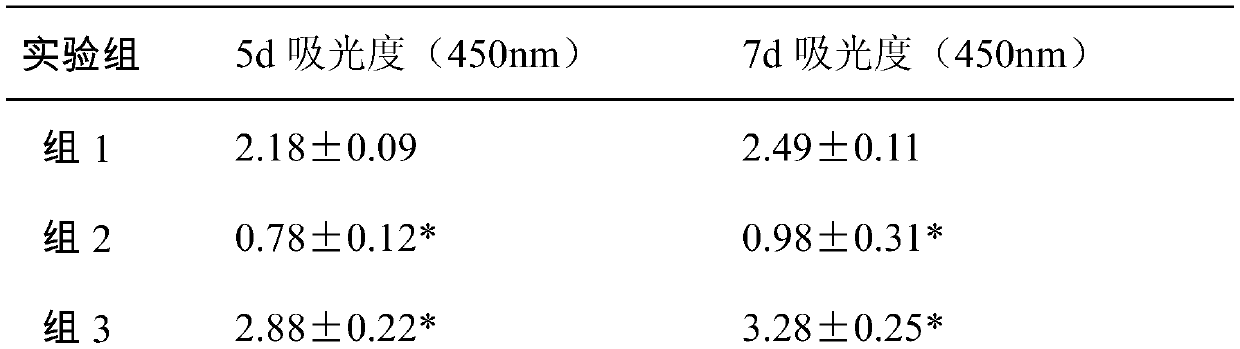
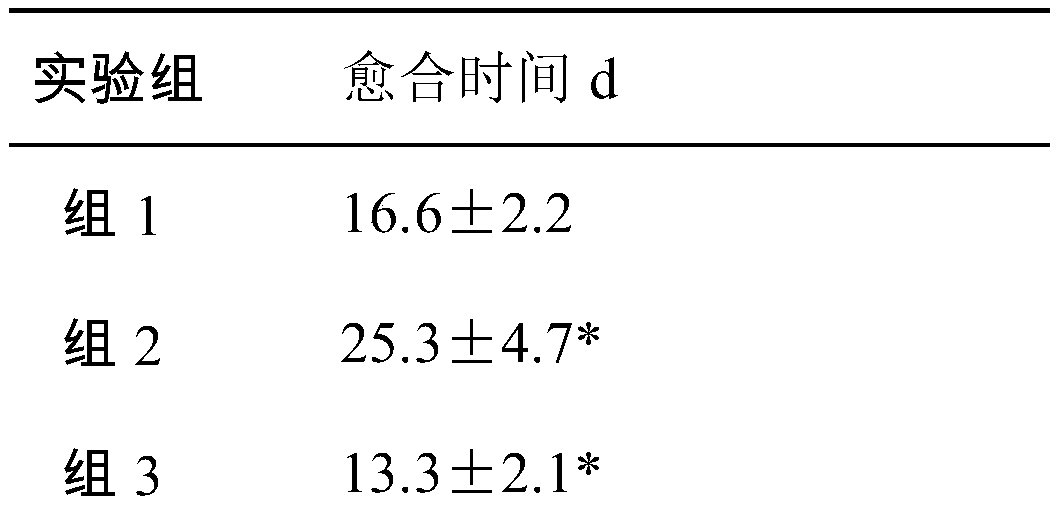
Laparotomy composite biological protection material
A technology of protecting material and abdominal cavity is applied in the field of composite biological protection material for abdominal cavity opening, which can solve the problem of no obvious improvement in late complications, and achieve the effects of delaying the occurrence time of immune rejection, reducing product antigenicity, and high social and economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] The preparation of embodiment 1 biological amnion
[0010] (1) The placenta obtained after delivery should be aseptically removed within 1 hour to remove the amniotic tissue;
[0011] (2) Use 0.6% glutaraldehyde solution to clean the blood and other impurities on the amniotic membrane, put it into a sterile PP bottle filled with 0.1% polysorbate 80 solution; put it in -40°C for at least 18 hours before use ;
[0012] (3) For production and use, thaw at 4°C for 20 hours;
[0013] (4) Take out the amniotic membrane in a sterile environment, put it into 400ml A solution and wash it 4 times;
[0014] (5) Spread the amniotic membrane on a sterile PP board to remove mucus in the basal layer;
[0015] (6) Soak for 60 minutes with a phosphate buffer solution of pH 7.0;
[0016] (7) Rub and wash with 0.6% glutaraldehyde solution for 30 minutes;
[0017] (8) Lay the amniotic membrane between two layers of sterile nylon mesh, and cut it to a suitable size;
[0018] (9) Pack ...
Embodiment 2
[0019] The preparation of embodiment 2 allogeneic dressings
[0020] (1) Take out the allogeneic skin raw materials that have passed the acceptance test and have been frozen at -80°C, and thaw them with 37°C CA solution in a Class 10,000 clean room, and then wash them twice with purified water for 25 minutes each time;
[0021] (2) 0.1% polysorbate 80 solution was treated 3 times, each time for 25 minutes, and then purified water was washed 2 times, each time for 25 minutes;
[0022] (3) Phosphate buffer solution of pH 7.0 was treated twice, each time for 25 minutes, and then purified water was washed twice, each time for 25 minutes;
[0023] (4) Washing twice with a 0.9% sodium chloride solution with a pH of 7.3, each time for 25 minutes;
[0024] (5) After adding an appropriate amount of 0.9% sodium chloride solution, double-layer sealed packaging; 30KGy irradiation sterilization.
Embodiment 3
[0025] Example 3 The use of open composite biological protection materials in the abdominal cavity
[0026] When the protective material is used, the biological amniotic membrane is applied near the innermost layer of the abdominal wall defect, and the allogeneic dressing is covered on it and sutured. According to the degree of injury, the biological amniotic membrane is set to 1-6 layers, and the heterogeneous dressing is set to a single layer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com