Compound with indoline skeleton, and preparation method and medical application of compound
A compound, indoline technology, used in organic chemistry, antipyretics, drug combinations, etc., can solve problems such as difficulty in achieving therapeutic selectivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
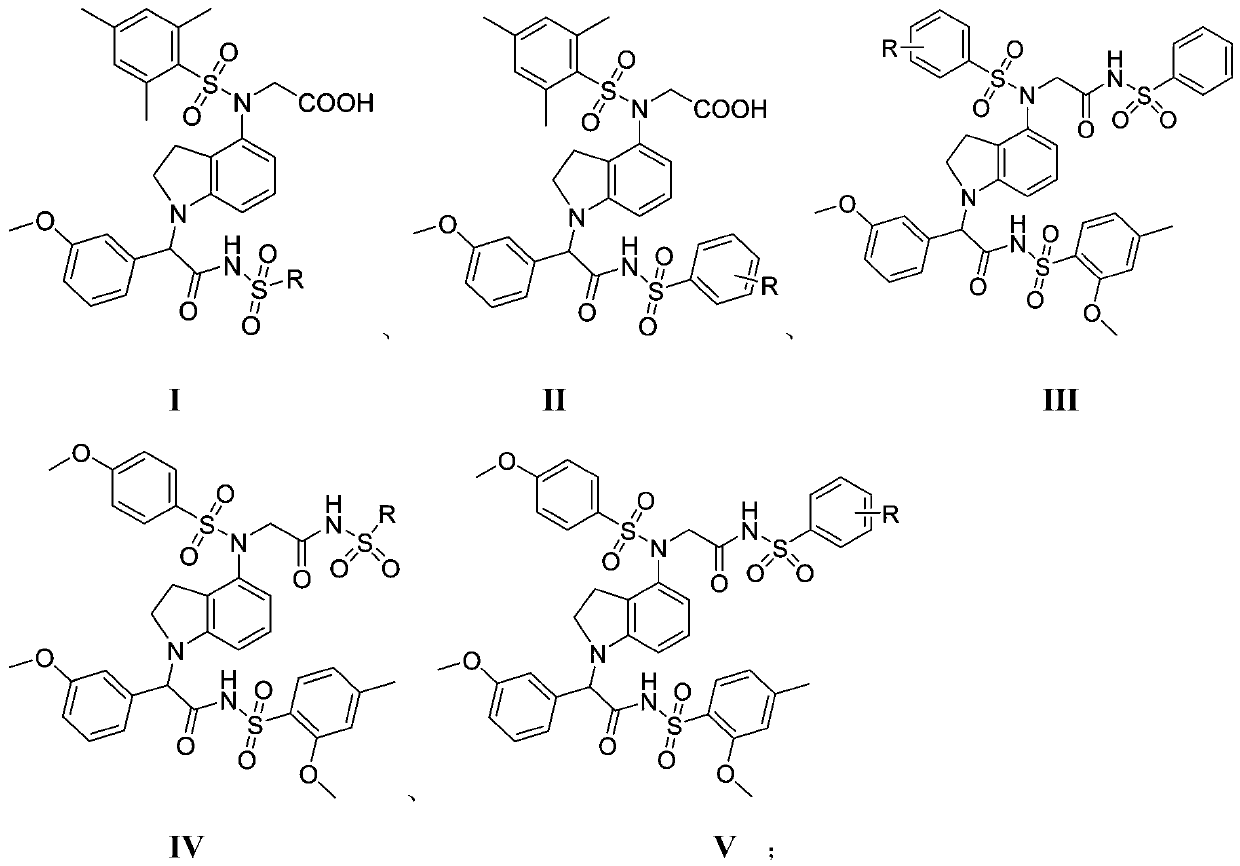
[0048] Example 1: N-(M-trimethylbenzenesulfonyl))-N-(1-(1-(3-methoxyphenyl)-2-oxo-2-(phenylsulfonyl)ethyl Base) indol-4-yl) glycine (S1)
[0049] (1) 4-nitroindoline (2)
[0050] Dissolve the raw material 4-nitroindole (3.0 g, 18.5 mmol) in 20.0 mL of DCM, add 6.0 mL of trifluoroacetic acid (TFA), add 4 times the equivalent of sodium cyanoborohydride in batches under ice bath, and stir at room temperature for 4 Hour. After the reaction, add saturated NaHCO 3 Adjust the pH to nearly neutral, wash the organic layer 3 times with a saturated NaCl solution, dry over anhydrous sodium sulfate, spin to dry the solvent, and obtain 2.4 g of a red solid by column chromatography with a yield of 79%; 1 HNMR (300MHz, Chloroform-d) δ7.14 (dd, J = 8.0, 1.3Hz, 1H), 7.04 (t, J = 8.0Hz, 1H), 6.65 (dd, J = 7.9, 1.3Hz, 1H), 3.34(t, J=5.5Hz, 2H), 2.94(t, J=6.5Hz, 2H); ESI-MS m / z: 165.1(M+H) + .
[0051] (2) Methyl 2-bromo-2-(3-methoxyphenyl)acetate (4)
[0052] Dissolve raw material 3 (3g, 1...
Embodiment 2
[0066] Example 2: N-(M-trimethylbenzenesulfonyl)-N-(1-(1-(1-(3-methoxyphenyl)-2-(methylsulfonyl)-2-oxo Ethyl)indol-4-yl)glycine (S2)
[0067]
[0068] The synthesis of S2 was the same as in Example 1, replacing benzenesulfonamide with methylsulfonamide to obtain 167.0 mg of a white solid with a yield of 68%; 1 H NMR (300MHz, DMSO-d 6 )δ12.88(s,2H),8.03(s,1H),7.82(d,J=1.8Hz,1H),7.49(t,J=7.9Hz,1H),6.99(d,J=7.2Hz, 1H), 6.91(s, 1H), 6.74(d, J=10.5Hz, 2H), 6.57(d, J=8.0Hz, 1H), 6.25(d, J=7.7Hz, 1H), 5.21(s, 1H), 4.28(s, 2H), 3.73(s, 3H), 3.26(d, J=8.5Hz, 1H), 2.94(s, 3H), 2.67(d, J=8.2Hz, 1H), 2.41( s,2H),2.21(s,9H); EI-MS HRMS(ESI):found616.1735(C 29 h 34 N 3 o 8 S 2 .[M+H]+requires 616.1746).
Embodiment 3
[0069] Example 3: N-(Mes-Trimethylbenzenesulfonyl)-N-(1-(1-(1-(3-methoxyphenyl)-2-oxo-2-(trifluoromethyl) Sulfonamido)ethyl)indol-4-yl)glycine (S3)
[0070]
[0071] The synthesis of S3 was the same as in Example 1, replacing benzenesulfonamide with trifluoromethanesulfonamide to obtain 142.0 mg of a white solid with a yield of 64%; 1 H NMR (300MHz, DMSO-d 6 )12.91(s,2H),δ8.05(s,1H),7.78(d,J=1.9Hz,1H),7.29(t,J=7.9Hz,1H),6.95(d,J=7.8Hz, 1H),6.90(s,1H),6.70(d,J=10.3Hz,2H),6.56(d,J=8.0Hz,1H),6.23(d,J=7.9Hz,1H),5.20(s, 1H), 4.29(s, 2H), 3.72(s, 3H), 3.25(d, J=8.5Hz, 1H), 2.66(d, J=8.2Hz, 1H), 2.40(s, 2H), 2.21( s,9H); EI-MSHRMS (ESI): found 670.1422 (C 29 h 31 f 3 N 3 o 8 S 2 .[M+H]+requires 670.1412).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com