Preparation method of anhydrous iron phosphate nanoparticles
A nanoparticle, iron phosphate technology, applied in the preparation/purification of carbon, nanotechnology, nanotechnology, etc., can solve the problems of high energy consumption and excessive excess
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 14.4236g concentration of 85wt% H 3 PO 4 The solution was added to 20 ml of deionized water and stirred to form a phosphoric acid solution. Add 2.7926g of Fe powder to the phosphoric acid solution, raise the temperature to 40°C, and stir for 0.5h. Add 2.5ml concentration of 30wt% H 2 o 2 (density 1.11g / ml) solution, continue stirring for 0.5h. Filter to obtain a tan filtrate. The obtained filter residue was dried and weighed, and the mass of the filter residue was 0.0514g, so the reaction degree of the iron powder in the first step was 98.15%. Add 70 ml of deionized water and 2.5 ml of 30 wt% H to the tan solution 2 o 2 The solution was stirred at room temperature for 1 h. Filter, wash, and dry to obtain light yellow powder. Make the SEM image of the light yellow powder, such as figure 1. The light yellow powder was placed in a muffle furnace, kept at 600°C for 6 hours, and 7.2480 g of light yellow powder was obtained after cooling. The calculated overall re...
Embodiment 2
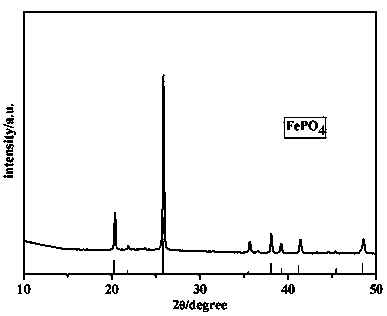
[0036] 7.9553g concentration of 85wt% H 3 PO 4 The solution was added to 20 ml of deionized water and stirred to form a phosphoric acid solution. Add 1.6754g of Fe powder to the phosphoric acid solution, raise the temperature to 40°C, and stir for 0.5h. Add 1.5ml of 30wt% H 2 o 2 Solution, continue to stir for 0.5h. Filter to obtain a tan filtrate. Add 75 ml of deionized water and 1.5 ml of 30% HO to the tan solution 2 o 2 The solution was stirred at room temperature for 1 h. Filter, wash, and dry to obtain light yellow powder. Place the light yellow powder in a muffle furnace at 700°C for 6 hours, and obtain a light yellow powder after cooling. The X-ray diffraction pattern of the powder proved to be pure phase FePO in crystalline state (PDF NO.29-0715) 4 .
Embodiment 3
[0038] 20.2917g concentration of 85wt% H 3 PO 4 The solution was added to 20 ml of deionized water and stirred to form a phosphoric acid solution. Add 4.4680g Fe powder to the phosphoric acid solution, raise the temperature to 40°C, and stir for 0.5h. Add 1.6ml of 30wt% H 2 o 2 Solution, continue to stir for 0.5h. Filter to obtain a tan filtrate. Add 68 ml of deionized water and 4.1 ml of 30% HO to the tan solution 2 o 2 The solution was stirred at room temperature for 1 h. Filter, wash, and dry to obtain light yellow powder. Place the light yellow powder in a muffle furnace at 600°C for 6 hours, and obtain a light yellow powder after cooling. The X-ray diffraction pattern of the powder proved to be pure phase FePO in crystalline state (PDF NO.29-0715) 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com