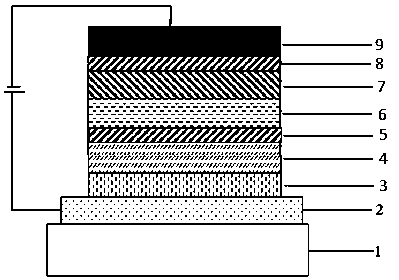
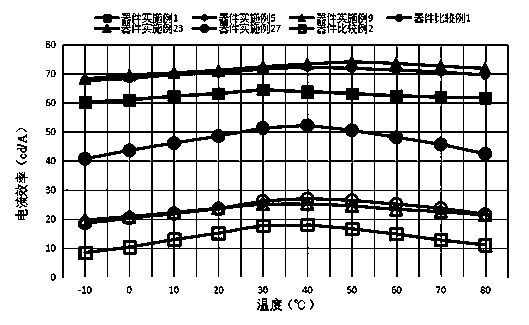
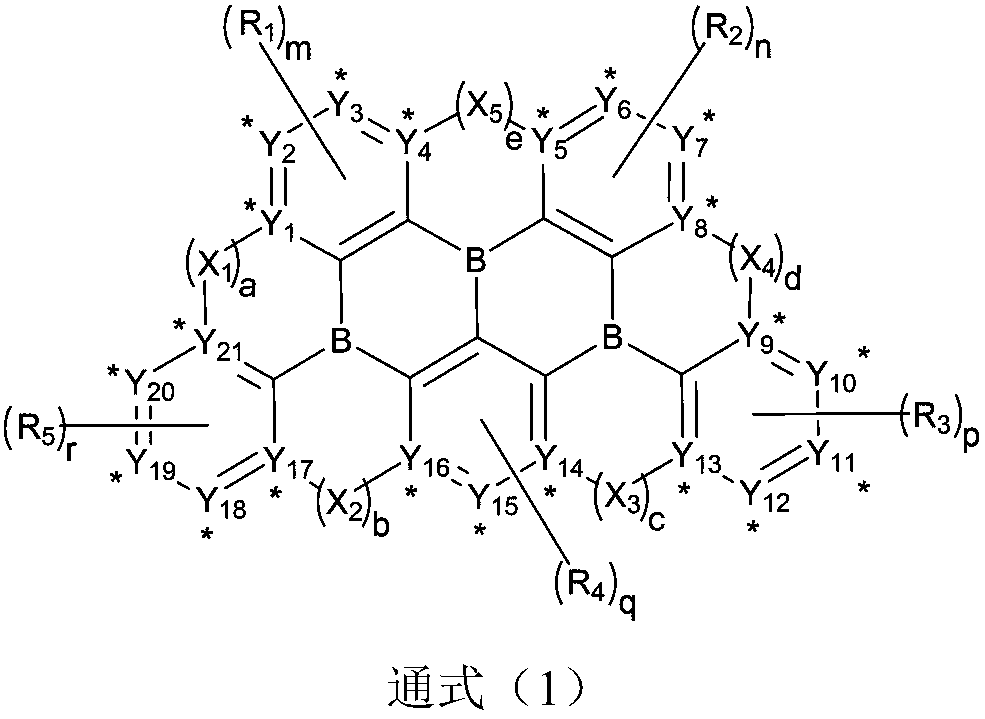
Boron-containing organic compound and application thereof in organic electroluminescent device
An organic compound and light-emitting layer technology, applied in the field of semiconductors, can solve problems such as efficiency roll-off, low S1 state radiation transition rate, difficult exciton utilization rate and high fluorescence radiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: the synthesis of intermediate G:
[0073] Take the synthesis of intermediate G1 as an example:
[0074]
[0075] In a 250mL three-neck flask, under a nitrogen atmosphere, add 0.025mol of raw material A-1, 0.01mol of raw material B-1, dissolve with a mixed solvent (90ml of toluene, 45ml of ethanol), and then add 0.03mol of Na 2 CO 3 aqueous solution (2M), stirred under nitrogen for 1 hour, then added 0.0001mol Pd(PPh 3 ) 4 , heating to reflux for 15 hours, sampling point plate, the reaction is complete. Naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain intermediate G-1 with a purity of 97.6% and a yield of 86.2%. Elemental analysis structure (molecular formula C 47 h 53 B 2 Cl): theoretical value C, 83.63; H, 7.91; B, 3.20; Cl, 5.25; found value: C, 83.63; H, 7.91; B, 3.21; Cl, 5.24. ESI-MS (m / z) (M+): The theoretical value is 674.40, and the measured value is 674.53.
[0076] In...
Embodiment 2
[0080] Embodiment 2: the synthesis of compound H1:
[0081]
[0082] (1) Weigh 0.04mol of raw material C-1 and 0.025mol of raw material D-1, cool down to -78°C; under an inert atmosphere, add 0.02mol of BCl 3 , heated to 100° C. and refluxed for 48 hours, the reaction was complete, and passed through a silica gel column to obtain intermediate J-1; the HPLC purity was 95.7%, and the yield was 74.6%.
[0083] Elemental analysis structure (molecular formula C 36 h 28 BrN 2 ): theoretical value C, 74.63; H, 4.87; B, 1.87; Br, 13.79; N, 4.84; test value: C, 74.63; ESI-MS(m / z)(M + ): The theoretical value is 578.15, and the measured value is 578.23.
[0084] (2) In a 250mL three-neck flask, under a nitrogen atmosphere, add 0.01mol of intermediate J-1, 0.015mol of raw material E-1, dissolve in a mixed solvent (90ml of toluene, 45ml of ethanol), and then add 0.03mol of Na 2 CO 3 aqueous solution (2M), stirred under nitrogen for 1 hour, then added 0.0001mol Pd(PPh 3 ) 4 , h...
Embodiment 3
[0087] Embodiment 3: the synthesis of compound H9:
[0088]
[0089] Take 0.1mol of intermediate G-1, add 0.12mol of tert-butyllithium, 120ml of tert-butylbenzene, keep warm at 60°C for 2 hours, cool down to room temperature, add dropwise 0.12mol of BBr 3 After fully reacting for half an hour, water was added to precipitate a solid, which was washed with n-hexane in sequence and recrystallized with ethanol to obtain compound H9. HPLC purity 96.3%, yield 81.7%.
[0090] Elemental analysis structure (molecular formula C 47 h 51 B 3 ): theoretical value C, 87.07; H, 7.93; B, 5.00; test value: C, 87.08; H, 7.92; B, 5.00. ESI-MS(m / z)(M + ): The theoretical value is 648.43, and the measured value is 648.51.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com