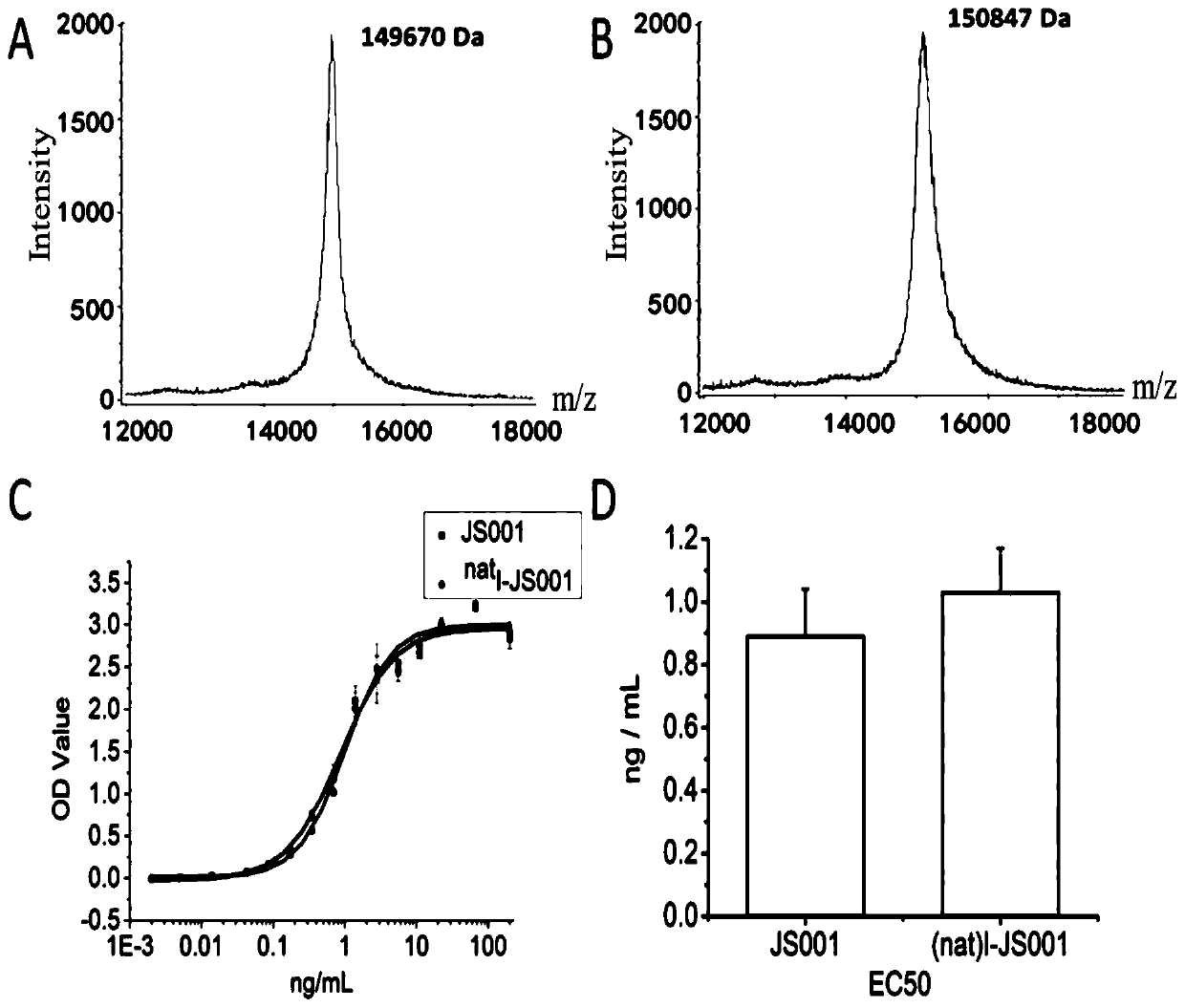
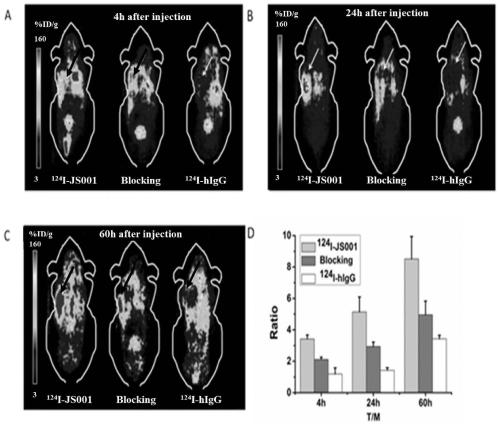
Nuclide-labeled PD-1-targeted monoclonal antibody and preparation method and application thereof
A monoclonal antibody, PD-1 technology, applied in the field of nuclear medicine, can solve the problems of inability to provide PD-1 and PD-L1 expression information, complex immunohistochemical detection, inability to monitor expression levels, etc., and achieve good in vitro stability. Sex, to achieve individualized, high-affinity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of novel radionuclide-labeled PD-1 targeting monoclonal antibody (1)
[0042] This example provides 124 I mark Toripalimab ( 124 I-Toripalimab) and preparation method thereof. 124 I-Toripalimab is prepared by NBS reaction, and the specific preparation method is as follows:
[0043] To 0.7ml, 45KBq / μL Na 124 Add 0.5ml of 0.1M PB buffer (pH7.2), 0.1mL, 10mg / mL of Toripalimab monoclonal antibody solution (prepared with water for monoclonal antibody solution) and 12 μg of N-bromosuccinimide (NBS) in the I solution , reacted at 37°C for 1min, and added 10% human serum albumin (HSA) to terminate the reaction; the reaction solution was purified by a PD-10 prepacked gel column to obtain the target product 124 I - Toripalimab.
[0044] Before use, the PD-10 prepacked gel column needs to be equilibrated with 0.01M PBS buffer of pH 7.4, add 5 mL each time, flow dry at a gravity flow rate, and repeat 5 times; then use 0.01M PBS buffer of pH 7.4 Purificat...
Embodiment 2
[0045] Example 2 Preparation of novel radionuclide-labeled PD-1 targeting monoclonal antibody (2)
[0046] This example provides 64 Cu marked Toripalimab ( 64 Cu-Toripalimab) and its preparation method. 64 Cu-Toripalimab is prepared through a bifunctional chelating agent coupling reaction, and the specific preparation method is as follows:
[0047] Add DFO equivalent to 6 times the molar equivalent of toripalimab monoclonal antibody to 1ml of toripalimab solution with a concentration of 2mg / ml, adjust the pH value of the reaction system to 8.5 with deionized 0.1M sodium bicarbonate solution; react at 37°C After 24 hours, the labeled precursor DFO-Toripalimab was obtained. Take 2 mg of the labeled precursor, add it to 1.0 ml of 0.1 M sodium acetate solution at pH 5.5, and then add 74 MBq of freshly prepared 64 Cu solution, adjust the pH to 7.0, and incubate at 40°C for 30 minutes; the resulting reaction solution is separated and purified by a PD-10 column to obtain the targ...
Embodiment 3
[0049] Example 3 Preparation of novel radionuclide-labeled PD-1 targeting monoclonal antibody (3)
[0050] This example provides 89 Zr marks Toripalimab ( 89 Zr-Toripalimab) and its preparation method. 89 Zr-Toripalimab is prepared through a bifunctional chelating agent coupling reaction, and the specific preparation method is as follows:
[0051] Add DFO equivalent to 6 times the molar equivalent of toripalimab monoclonal antibody to 1ml of toripalimab solution with a concentration of 2mg / ml, adjust the pH value of the reaction system to 8.5 with deionized 0.1M sodium bicarbonate solution; react at 37°C After 24 hours, the labeled precursor DFO-Toripalimab was obtained. Take 2 mg of the labeled precursor, add it to 1.0 ml of 0.1 M sodium acetate solution at pH 5.5, and then add 74 MBq of freshly prepared 89 Zr solution, adjust the pH to 7.0, and incubate at 40°C for 30 minutes; the resulting reaction solution is separated and purified by a PD-10 column to obtain the targe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com