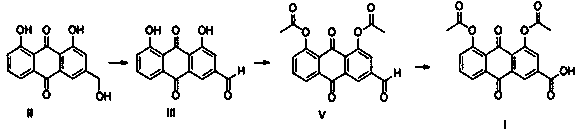
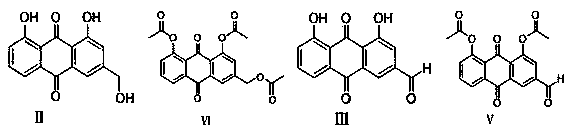
Diacerein synthesis method based on anthraquinone medicines
A technology of diacerein and synthesis method, which is applied in the field of diacerein synthesis, can solve the genotoxic impurity of aloe-emodin triacetylaloe-emodin. There is no economical and effective method suitable for industrial production, and the impurity removal control process is not related. Literature reports and other issues, to achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]
[0052] Add 2kg N-methylpyrrolidone and 200g aloe-emodin to the reaction bottle, heat up to 70°C, add 230g IBX, then stir and keep warm for 2h; Wash, drain, and vacuum-dry at 80°C for 12 hours to obtain 181 g of rhein;
[0053] Feed 181g rhubaraldehyde and 2400g dichloromethane into the reaction bottle, add 160g acetyl chloride at 15°C, keep 15°C, add 280g triethylamine dropwise within 30 minutes, stir for 10min after the dropwise addition, and then stir the reaction at room temperature 1h; after the reaction, the feed liquid was rotary evaporated at 35°C, evaporated to dryness, added 350g of methanol and 350g of water, beat at room temperature for 0.5h, filtered with suction, and vacuum-dried the filter cake at 80°C for 8h to obtain 205g of diacetyl rhein as a solid;
[0054] Add 205g of diacetyl rhein, 1900g of acetone, and 570g of DMSO in the reaction flask, and add 450g of NaH dropwise within 30 minutes under stirring. 2 PO 4 2H 2 A solution of O mixed with 1...
Embodiment 2
[0060] On the basis of Example 1, the amount of all raw materials was increased by 100 times, and the rest remained unchanged to obtain a refined product of diacerein with a total yield of 51.6% and a purity of 99.72%; the obtained diacerein sample was tested by a genotoxicity method. The residue of aloe-emodin impurities is 11ppm, and the residue of rhein-type impurities is 8ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com