Method for preparing catalyst through solid-state electroreduction
A catalyst and solid-state technology, which is applied in the field of solid-state electroreduction to prepare catalysts, can solve the problem of not being able to solve the problem of one-time large-scale preparation of homogeneous catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
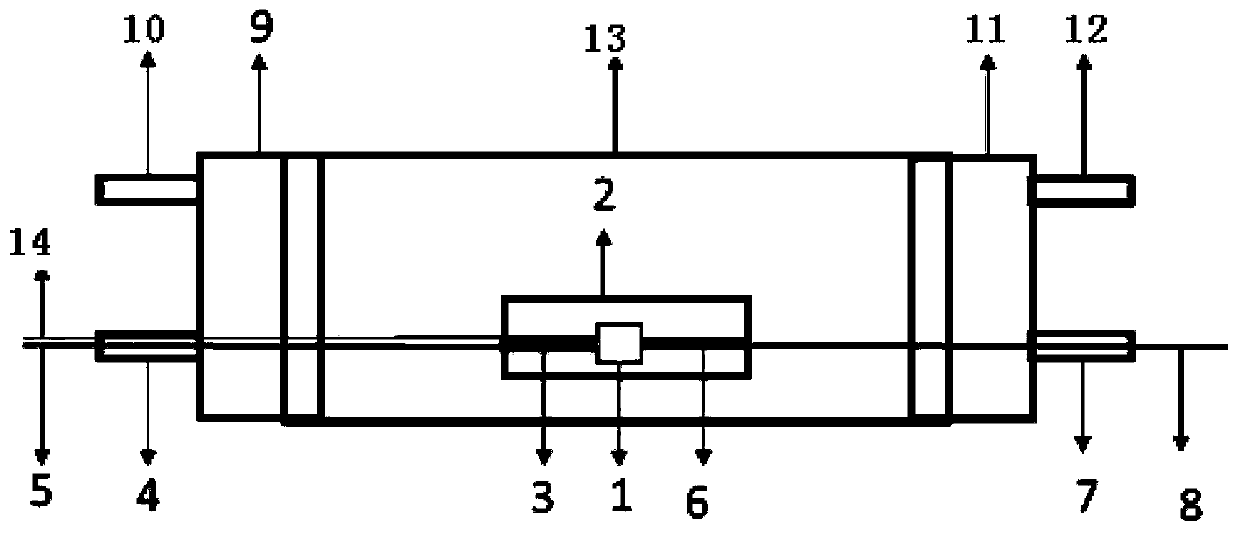
[0025] The reaction device for preparing catalyst of the present invention is as figure 1 As shown, the device includes an insulating tube 13, the two ends of the insulating tube 13 shown are respectively connected to the air inlet port 9 and the gas outlet port 11, and one side of the air inlet port 9 is provided with an air inlet and a positive lead wire inlet, and the air inlet and The positive lead wire inlet is respectively connected to the air intake pipe 10 and the positive lead wire tube 4, and the air outlet port 11 side is provided with an air outlet and a negative electrode lead wire inlet, and the gas outlet and the negative electrode lead wire inlet are respectively connected to the air outlet pipe 12 and the negative electrode lead wire tube 7;
[0026] The insulating tube 13 is provided with a sample tank 2 for placing the sample 1. The two ends of the sample 1 are respectively connected to the positive electrode 3 and the negative electrode 6. The positive elect...
Embodiment 1
[0037] Take 100mg carbon black material (BP), add 30ml 6M HNO 3 , ultrasonic dispersion for 1h, adding 20mg of Pt ions (H 2 Cl 6 Pt solution is the Pt source), stirred in an oil bath at 80°C for 6h. Excess solvent was rotovapped to give a solid powder.
[0038] Take 50mg of solid powder and put it in the sample tank, put the sample tank into the insulating tube, vacuumize the device, remove the air in the reaction system, and then pass the N 2 , and keep N 2 Environment, the two ends of the sample are respectively connected to the positive and negative poles of the constant current and constant voltage power supply, the sample is subjected to 1.5A constant current treatment for 5 hours, and then the sample is washed with deionized water and ethanol several times, and dried in a vacuum oven at 60°C for 24 hours .
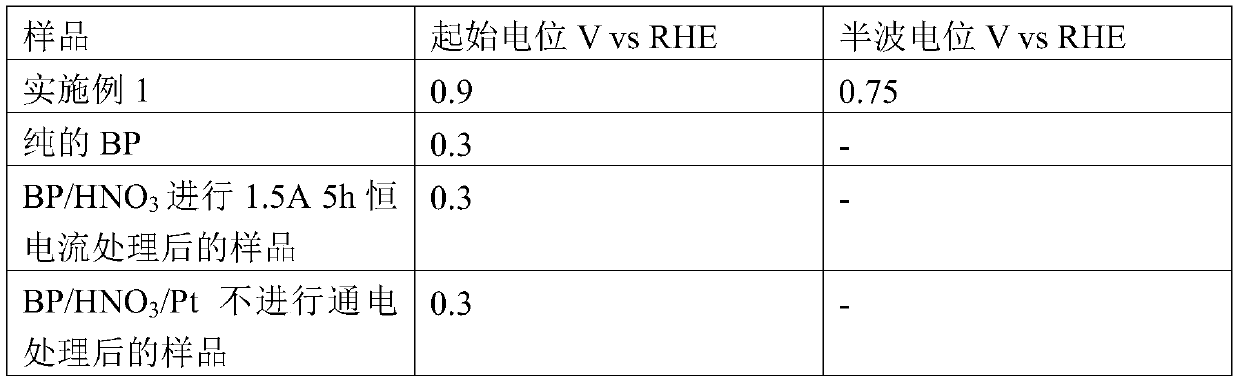
[0039] Carry out oxygen reduction test to the sample that embodiment 1 obtains, simultaneously to blank sample, comprise pure BP, BP / HNO 3 Sample after 1.5A 5h...
Embodiment 2
[0044] Take 100mgBP, add 30ml 6M HNO 3 , ultrasonically dispersed for 1 h, then refluxed at 80°C for 6 h, rotary evaporated, and vacuum-dried to obtain BPox treated with nitric acid. Then mix 100mg BPox with 300ml 5wt.% FeCl 3 Aqueous solution (containing FeCl 3 15.26mg) were ultrasonically mixed and dried to obtain BPox-Fe.
[0045] BPox-Fe-Urea was obtained by grinding 100 mg BPox-Fe with 500 mg urea. Put the BPox-Fe-Urea powder compact into the sample tank, put the sample tank into the insulating tube, and pass through the N 2, the two ends of the sample are respectively connected to the positive and negative poles, and a voltage pulse (+10V 10s, -10V 10s, 20V 10s, -20V 10s) is applied to it. Then the samples were washed and dried to obtain BP-NFe.
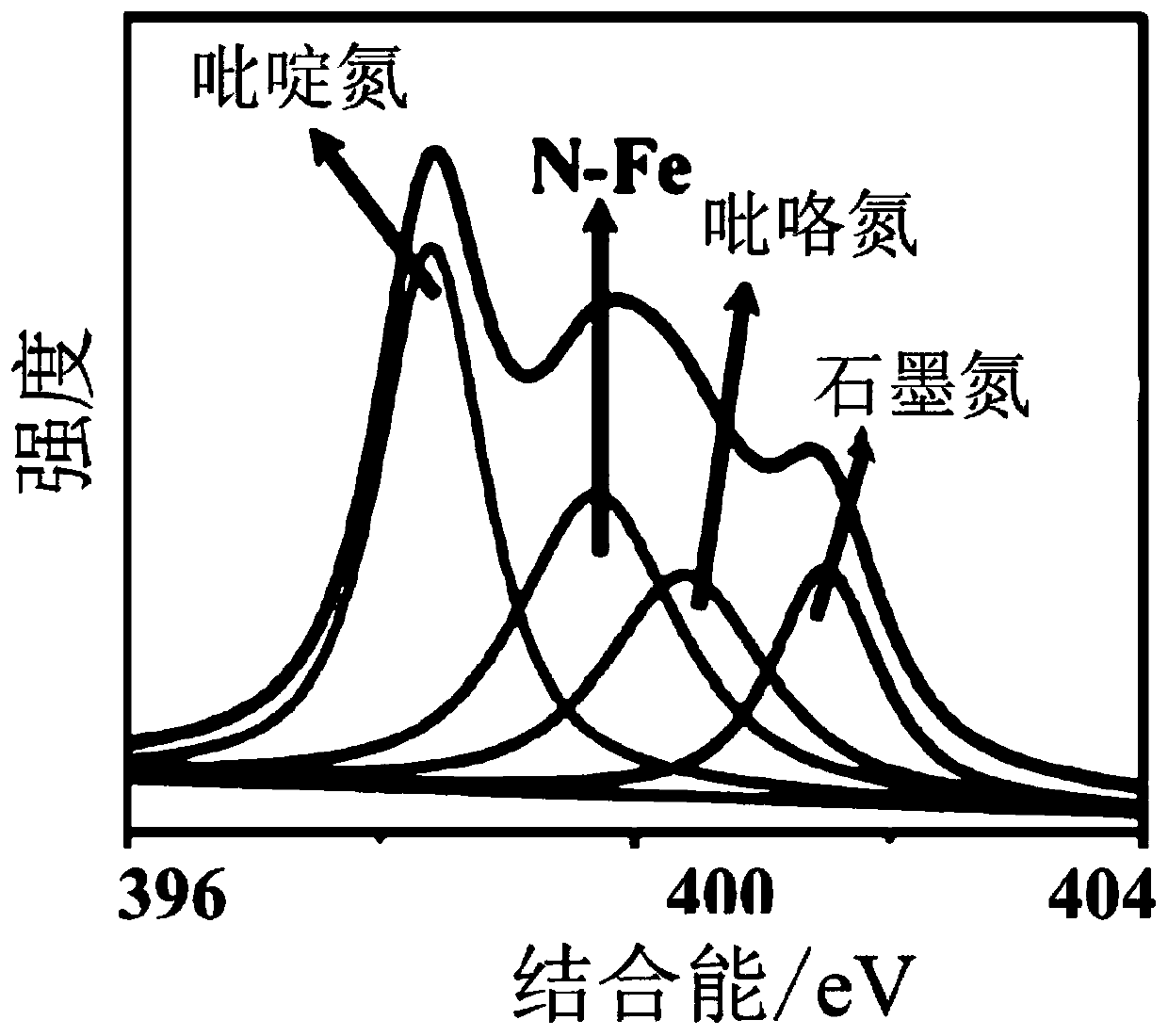
[0046] By characterizing the chemical valence state of the catalyst of Example 2, it forms a Fe-N-C structure, such as figure 2 The XPS results are shown. By measuring the oxygen reduction performance of the catalyst u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com