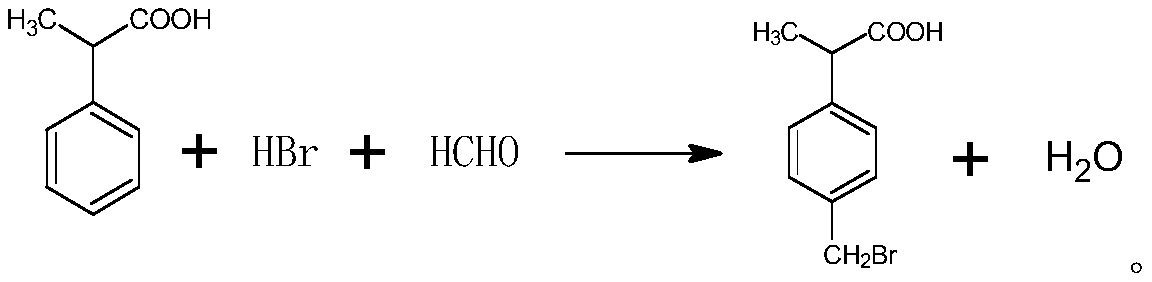
Preparation method of p-bromomethyl isophenylpropionic acid
A technology of p-bromomethylisophenylpropionic acid and phenylpropionic acid, which is applied in the field of synthesis of pharmaceutical intermediates, and can solve the problem of large-scale industrial production of bromomethylisophenylpropionic acid and the difficulty of obtaining 4-methylstyrene , high cost of 4-methylstyrene, etc., to achieve the effect of easy purchase, low price and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
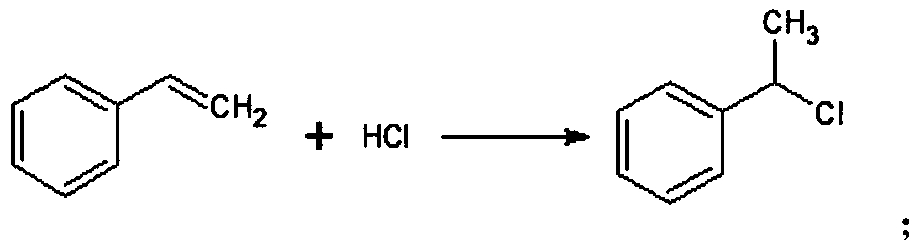
[0045] Synthesis of A, α-methylbenzyl chloride
[0046] Put 300g of dichloromethane and 100g of styrene into the addition kettle, control the temperature at -5 ~ 5°C, and continuously feed dry hydrogen chloride to react for 10 hours; after the reaction, blow off the residual hydrogen chloride with nitrogen; then distill at atmospheric pressure Remove dichloromethane, high vacuum rectification obtains 133g α-methyl benzyl chloride, after testing, the purity of α-methyl benzyl chloride is 98.3%, and the yield is 98.1%;
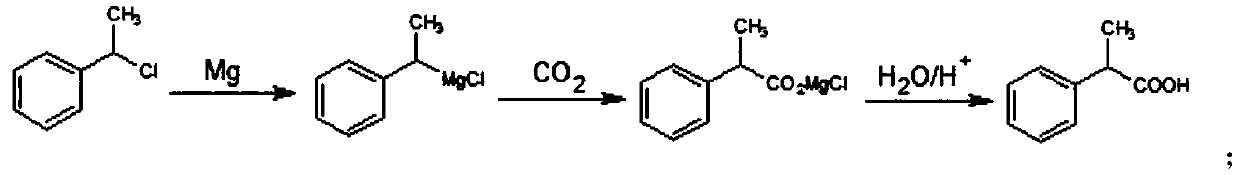
[0047] Synthesis of B, 2-phenylpropionic acid
[0048] Dissolve 133g of α-methylbenzyl chloride in 665g of 2-methyltetrahydrofuran, add magnesium powder with a molar ratio (calculated as α-methylbenzyl chloride) of 2 times, and react at 10-20°C for 3 hours to prepare a Grignard solution; Control 5-15°C, pass through dry carbonic acid gas with a molar ratio of 1.5 times, and react for 5 hours to obtain a carboxylated solution. After the reaction was finished, 2...
Embodiment 2
[0052] Synthesis of A, α-methylbenzyl chloride
[0053] Put 200g of dichloromethane and 100g of styrene into the addition kettle, control the temperature at 0-10°C, and continuously feed dry hydrogen chloride to react for 15 hours; after the reaction, blow off the residual hydrogen chloride with nitrogen; Except dichloromethane, high vacuum rectification obtains 128g α-methyl benzyl chloride, after testing, the purity of α-methyl benzyl chloride is 98.0%, and the yield is 94.4%;
[0054] Synthesis of B, 2-phenylpropionic acid
[0055]Dissolve 128g of α-methylbenzyl chloride in 512g of 2-methyltetrahydrofuran, add magnesium powder with a molar ratio of 1.8 times (in terms of α-methylbenzyl chloride), and react at 0-10°C for 10 hours to prepare a Grignard solution; Controlling -15~-5℃, passing through dry carbonic acid gas with a molar ratio of 1.5 times, and reacting for 5 hours to obtain a carboxylated solution. After the reaction was finished, 2-methyltetrahydrofuran was re...
Embodiment 3
[0059] Synthesis of A, α-methylbenzyl chloride
[0060] Put 400g of dichloromethane and 100g of styrene into the addition kettle, control the temperature at 10-20°C, and continuously feed dry hydrogen chloride to react for 5 hours; after the reaction, blow off the residual hydrogen chloride with nitrogen; Except dichloromethane, high vacuum rectification obtains 130g α-methyl benzyl chloride, after testing, the purity of α-methyl benzyl chloride is 98.1%, and the yield is 95.9%;
[0061] Synthesis of B, 2-phenylpropionic acid
[0062] Dissolve 130g of α-methylbenzyl chloride in 780g of 2-methyltetrahydrofuran, add magnesium powder at a molar ratio of 2.2 times (in terms of α-methylbenzyl chloride), and react at 20-30°C for 5 hours to prepare a Grignard solution; Control 20-30°C, pass through dry carbonic acid gas with a molar ratio of 1.5 times, and react for 5 hours to obtain a carboxylated solution. After the reaction was finished, 2-methyltetrahydrofuran was removed by at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com