Method for preparing intravenous injection human immune globulin from plasma component FI + III precipitates
A technology of human immunoglobulin and plasma, applied in the field of preparation of intravenous human immunoglobulin, which can solve the problem that the high content of activated coagulation factors cannot guarantee product quality, increase production cost and operation complexity, and increase the risk of product contamination, etc. problems, to achieve the effect of improving comprehensive utilization, shortening production cycle, and reducing blood coagulation risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for preparing intravenous human immunoglobulin from the precipitation of plasma components FI+III:
[0031] ①Preparation of component FI+III precipitation
[0032] Add a certain concentration of ethanol from the qualified human plasma to separate the FI+II+III precipitation. After the FI+II+III precipitation is dissolved, add ethanol to a concentration of 14%, adjust the pH to 5.20, and set the temperature to -4°C. Diatomite and perlite, FI+III wastes are filtered out by pressure filtration, and the component II is dissolved and purified by chromatography to prepare human immunoglobulin.
[0033] ②Component FI+III precipitation and dissolution
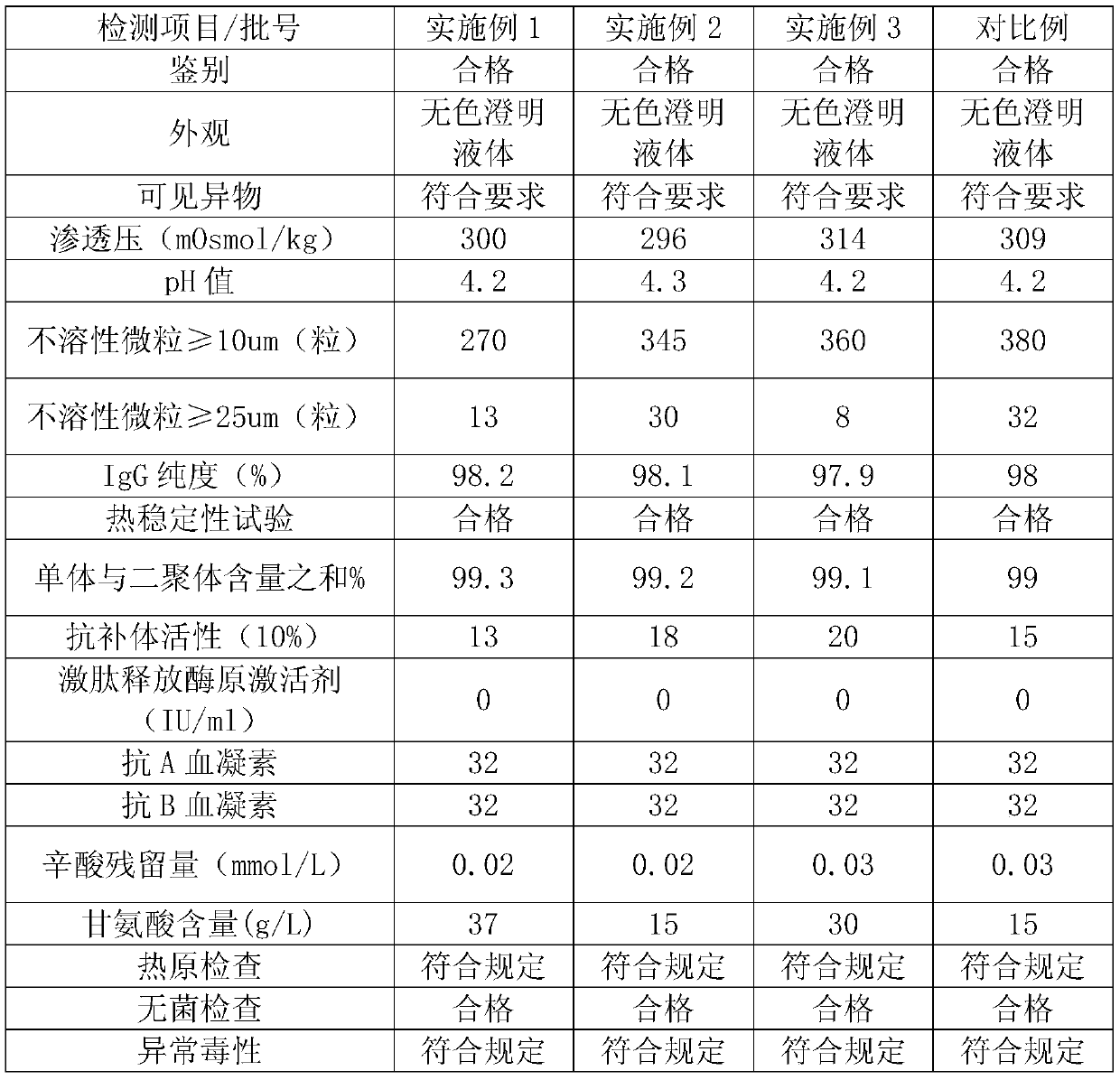
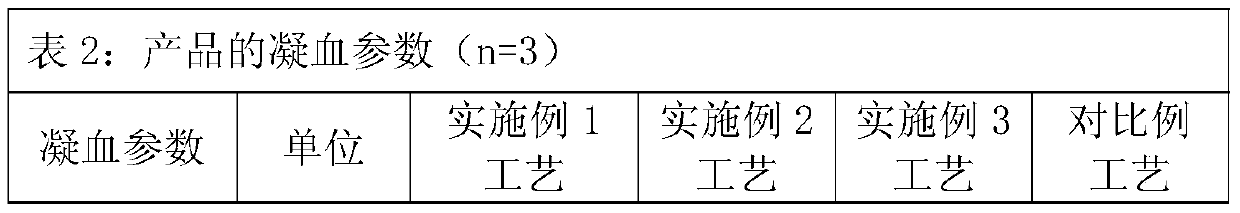
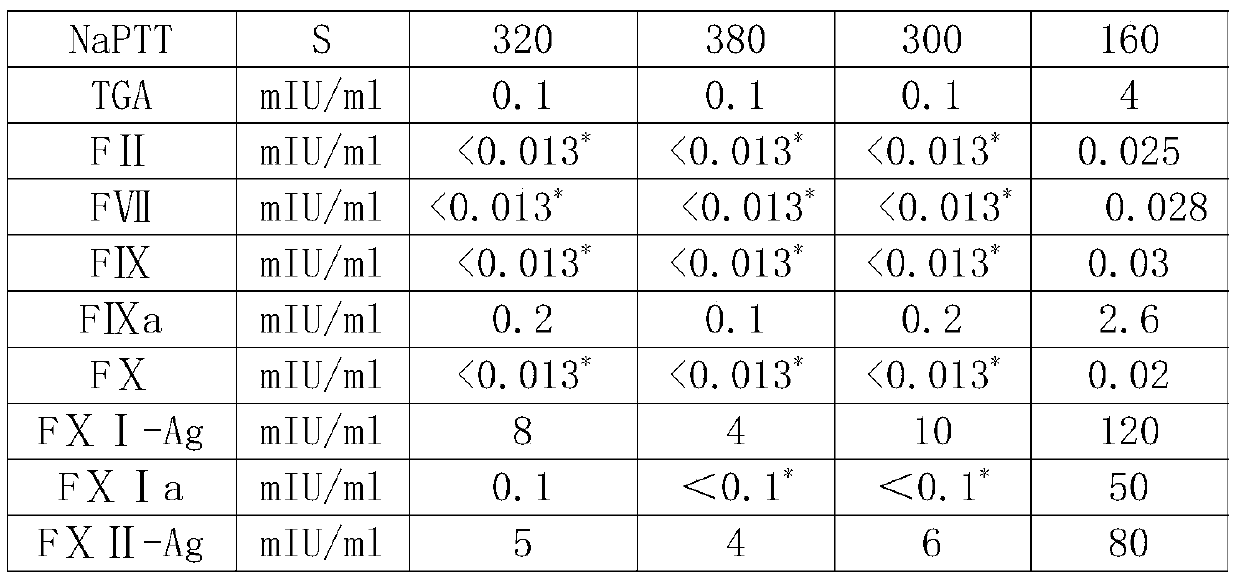
[0034] The precipitation solution is 20mmol / L phosphate buffer, adjust the pH value to 4.9 with acetic acid, and after putting in 10kgFI+III precipitation, the dissolution rate is 10 times, and the IgG concentration is 1.4g / L (for IgG detection, refer to the 2015 edition of the Chinese Pharmacopoeia Four General Rules ...
Embodiment 2
[0048] A method for preparing intravenous human immunoglobulin from the precipitation of plasma components FI+III:
[0049] ①Preparation of component FI+III precipitation
[0050] A certain concentration of ethanol was added to the qualified human plasma to separate the FI+II+III precipitate. After the FI+II+III precipitate was dissolved, the concentration of ethanol was added to 14%, the pH was adjusted to 5.24, and the temperature was -4°C. Diatomite and perlite, FI+III wastes are filtered out by pressure filtration, and the component II is dissolved and purified by chromatography to prepare human immunoglobulin.
[0051] ②Component FI+III precipitates and dissolves
[0052] The precipitation solution is 10mmol / L phosphate buffer, adjust the pH value to 4.0 with acetic acid, and after putting in 10.5kg FI+III precipitation, the dissolution rate is 5 times, and the IgG concentration is 2.8g / L (for IgG detection, refer to Part Four of the Chinese Pharmacopoeia of the 2015 edi...
Embodiment 3
[0066] A method for preparing intravenous human immunoglobulin from the precipitation of plasma components FI+III:
[0067] ①Preparation of component FI+III precipitation
[0068] A certain concentration of ethanol was added to the qualified human plasma to separate the FI+II+III precipitate. After the FI+II+III precipitate was dissolved, the concentration of ethanol was added to 14%, the pH was adjusted to 5.24, and the temperature was -4°C. Diatomite and perlite, FI+III wastes are filtered out by pressure filtration, and the component II is dissolved and purified by chromatography to prepare human immunoglobulin.
[0069] ②Component FI+III precipitates and dissolves
[0070] The precipitation solution is 15mmol / L phosphate buffer, adjust the pH value to 4.5 with acetic acid, and after putting in 11.0kg FI+III precipitation, the dissolution rate is 7 times, and the IgG concentration is 1.99g / L (for IgG detection, refer to the fourth part of the Chinese Pharmacopoeia of the 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com