Immune effector cell for chronic lymphocytic leukemia as well as preparation method and application thereof
A technology of immune effector cells and proteins, applied in the field of medical biology, can solve problems such as complexity, difficulty, and unsuitability for targeted therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1, Construction of the Expression Vector of CD32b CAR Molecule
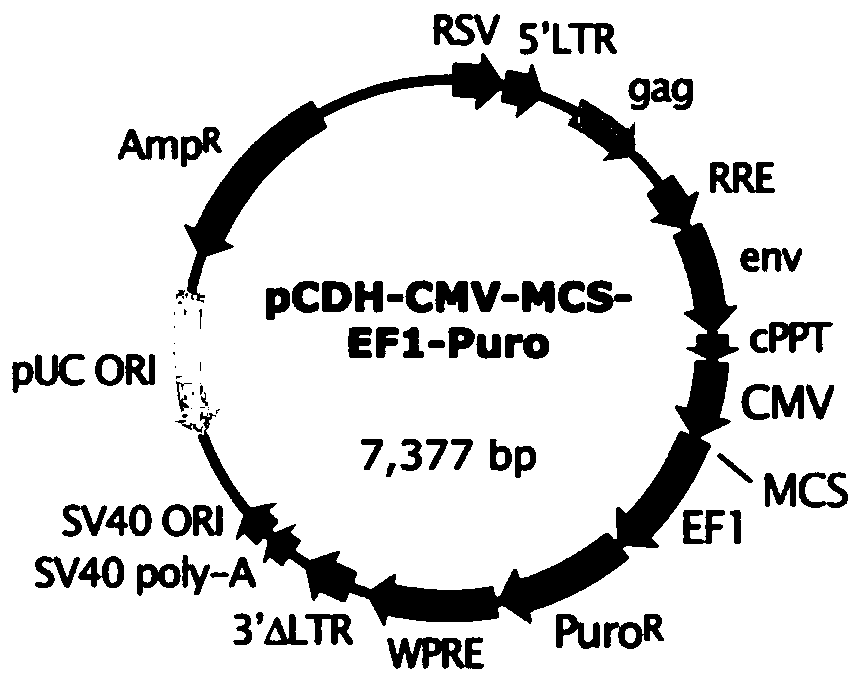
[0100] In this example, a lentiviral vector expressing CAR molecules is constructed, and the lentiviral vector pCDH is used as the backbone plasmid, and the structure of pCDH is as follows: figure 2 shown.
[0101] According to the difference of the CAR molecule scFv, the inventors constructed two kinds of CD32b CAR molecules, namely scFv1-BBZ and scFv2-BBZ: scFv1 and scFv2 are CD32b humanized antibodies. The molecular structure patterns of scFv1-BBZ and scFv2-BBZ are shown in figure 1 shown. The above CAR molecule was constructed into the lentiviral vector pCDH by conventional molecular cloning means.
[0102] Construction of scFv1-BBZ vector: First, scFv1-BBZ nucleic acid fragment was chemically synthesized into pUC57 vector (Beijing Huada Protein Biology Co., Ltd.), and scFv1-BBZ fragment (SEQ ID NO: 11) was obtained by XmaI / SalI double enzyme digestion. Wherein, scFv1 sequence such as SEQ ...
Embodiment 2
[0109] Example 2, Construction of CD32b CAR-T cells
[0110] In this example, the inventors constructed CD32b CAR-T cells.
[0111] Packaging of CAR lentivirus: First, use the MACHEREY-NAGEL endotoxin-free large-scale extraction plasmid kit to extract the lentiviral plasmids pCDH-scFv1-BBZ, pCDH-scFv2-BBZ and pCDH (without CD32b CAR control empty vector) constructed in Example 1 Plasmids) and lentiviral system auxiliary packaging plasmids Rev, VSV-G and pMDL. The day before transfection, 1×10 7 A 293T plate was placed in a T75 culture flask. 20 minutes before transfection, the medium of 293T cells was replaced with 5ml of serum-free DMEM medium. The plasmids were co-transfected into 293T cells using PEI transfection reagent, and the cell culture medium was replaced with 10 ml complete medium DMEM+10% FBS 6 hours after transfection. Cell supernatant was harvested 48 hours after transfection, and 10 ml of fresh complete medium was added. After 72 hours, the cell supernatant...
Embodiment 3
[0114] Example 3, Determination of CD32b CAR-T cell activity
[0115] In this example, the activity of CD32b CAR-T cells was measured.
[0116] 1. Identification and construction of CD32b CAR-T target cells
[0117] Human chronic lymphocytic leukemia Mec-1 cell line and Burkitt's lymphoma Raji cell line were used to detect the expression of CD32b on the two cell lines using CD32b-APC flow cytometry antibody.
[0118] The result is as Figure 5 As shown, the Raji cell line highly expresses the CD32b gene. Using a lentiviral vector encoding the GFP-Luciferase gene, the GFP-positive cells were sorted to construct a Raji cell line with a high expression of the GFP-Luciferase gene. CD32b and GFP-Luciferase expression levels in Raji cell lines see Figure 5 .
[0119] according to Figure 5 , Raji cell line is positive for CD32b expression.
[0120] 2. Cytokine secretion experiment
[0121] In a 96-well plate, scFv1-BBZ CAR-T cells or control pCDH T cells (effector cells) we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com