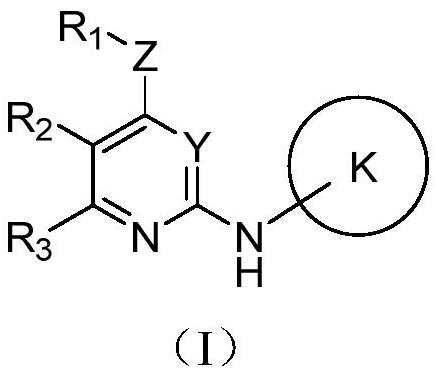
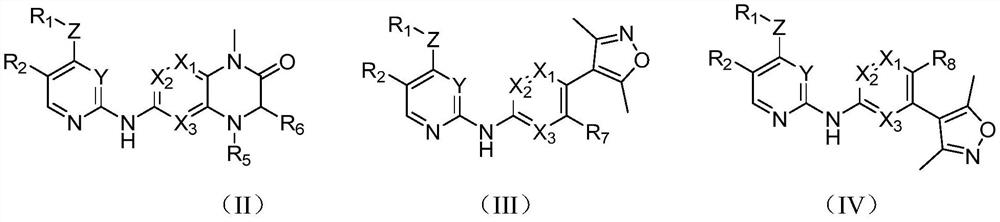
Compound with multi-target inhibition effect, composition, functional molecule and application of compound
A compound and ring atom technology, applied in the field of multi-target inhibitory compounds, can solve problems such as poor therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0133] The present invention also provides a preparation method of the above-mentioned compound, comprising the following steps:
[0134] S101: providing a compound of the structure shown in formula (I-1);
[0135]
[0136] Wherein, P represents halogen;
[0137] Wherein, the definition of each group in the structural compound shown in formula (I-1) is as above, and will not be repeated here.
[0138] S102: providing a compound of structure shown in formula (I-2);
[0139]
[0140] The definition of each group in the structural compound shown in formula (I-2) is as above, and will not be repeated here.
[0141] Further, step S102 includes the following steps:
[0142] S1021: making compound A and amino acid NH 2 R 1 CHCOOH is reacted to obtain compound B;
[0143] S1022: Compound B is subjected to reduction and ring closure with hydrosulfite to obtain compound C;
[0144] S1023: Reductive amination of compound C with the corresponding ketone to obtain compound D; ...
Embodiment 1
[0195]
[0196] (a) Compound 1A (25g, 113.64mmol), D-alanine (11.12g, 125.0mmol), potassium carbonate (17.25g, 125.0mmol) were dissolved in 500ml of ethanol: water = 3: 1 mixed solvent, 80 Heat to reflux at ℃ for 8 hours, monitor the reaction with a TLC plate, cool to room temperature after the reaction, evaporate the solvent, dissolve in water, adjust the pH to 1-2 with 1N HCl, a large amount of yellow solid precipitates, filter, and wash with 200ml petroleum ether The solid was dried in a vacuum oven to obtain 28.7 g of a yellow solid, that is, compound 1B, with a yield of 88%.
[0197] 1 H NMR (400MHz, CDCl 3 )δ8.35(d, J=6.9Hz, 1H), 8.06(d, J=9.1Hz, 1H), 6.90(s, 1H), 6.85(d, J=9.2Hz, 1H), 4.33(p, J=7.0Hz, 1H), 1.67 (d, J=7.0Hz, 3H).
[0198] (b) Compound 1B (28.7g, 99.31mmol), potassium carbonate (27.41g, 198.62mmol) was dissolved in 500ml of water, sodium dithionite (86.45g, 496.55mmol) was slowly added in batches, and reacted at 60°C for 8h to obtain colorless The ...
Embodiment 2
[0217]
[0218] The compound of Example 2 was prepared in the same manner as in Example 1, except that pyridine-3-boronic acid was used instead of phenylboronic acid in step (j) of Example 1.
[0219] 1 H NMR (400MHz, DMSO-d 6 )δ9.67(s, 1H), 9.35(d, J=2.2Hz, 1H), 8.74(dd, J=4.8, 1.6Hz, 1H), 8.59(d, J=5.2Hz, 1H), 8.49( dt, J=8.0, 2.0Hz, 1H), 7.63–7.54(m, 2H), 7.47(d, J=5.1Hz, 1H), 7.30(dd, J=8.7, 2.1Hz, 1H), 7.03(d ,J=8.7Hz,1H),4.11(q,J=6.7Hz,1H),3.89(p,J=6.6Hz,1H),3.26(s,3H),1.28(d,J=6.6Hz,3H ), 1.21(d, J=6.5Hz, 3H), 0.99(d, J=6.7Hz, 3H).LC-MS(ESI)[M+H] + :388.22.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com