Preparation method and application of inorganic colloidal electrolyte of aqueous zinc ion battery
A technology of zinc-ion batteries and inorganic colloids, applied in the direction of electrolyte immobilization/gelation, secondary batteries, circuits, etc., can solve problems such as reducing ion conductivity and battery life, not suitable for practical applications, and polymer structure reorganization , to achieve good biocompatibility and thermal stability, easy to implement, and increase the diffusion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Solution configuration: ① Dissolve zinc sulfate in deionized solution to prepare 2mol / L zinc sulfate; ② Dissolve zinc sulfate and manganese sulfate in deionized water to prepare 2mol / L zinc sulfate + 0.1mol / L manganese sulfate liquid electrolyte.
[0037]Add calcium hydroxyphosphate into 2mol / L zinc sulfate solution at a mass ratio of 3:10. After mixing, let it stand for 12 hours to allow sufficient calcium-zinc ion exchange reaction to occur, and then carry out suction filtration. Drying for 12 hours under the hood to obtain inorganic powder;
[0038] Grind the inorganic powder into a fine powder in a mortar, add it into the liquid electrolyte according to the fact that the inorganic powder accounts for 60% of the mass of the electrolyte, mix evenly, and press it into a sheet with a thickness of 0.5 mm to obtain the inorganic colloidal electrolyte.
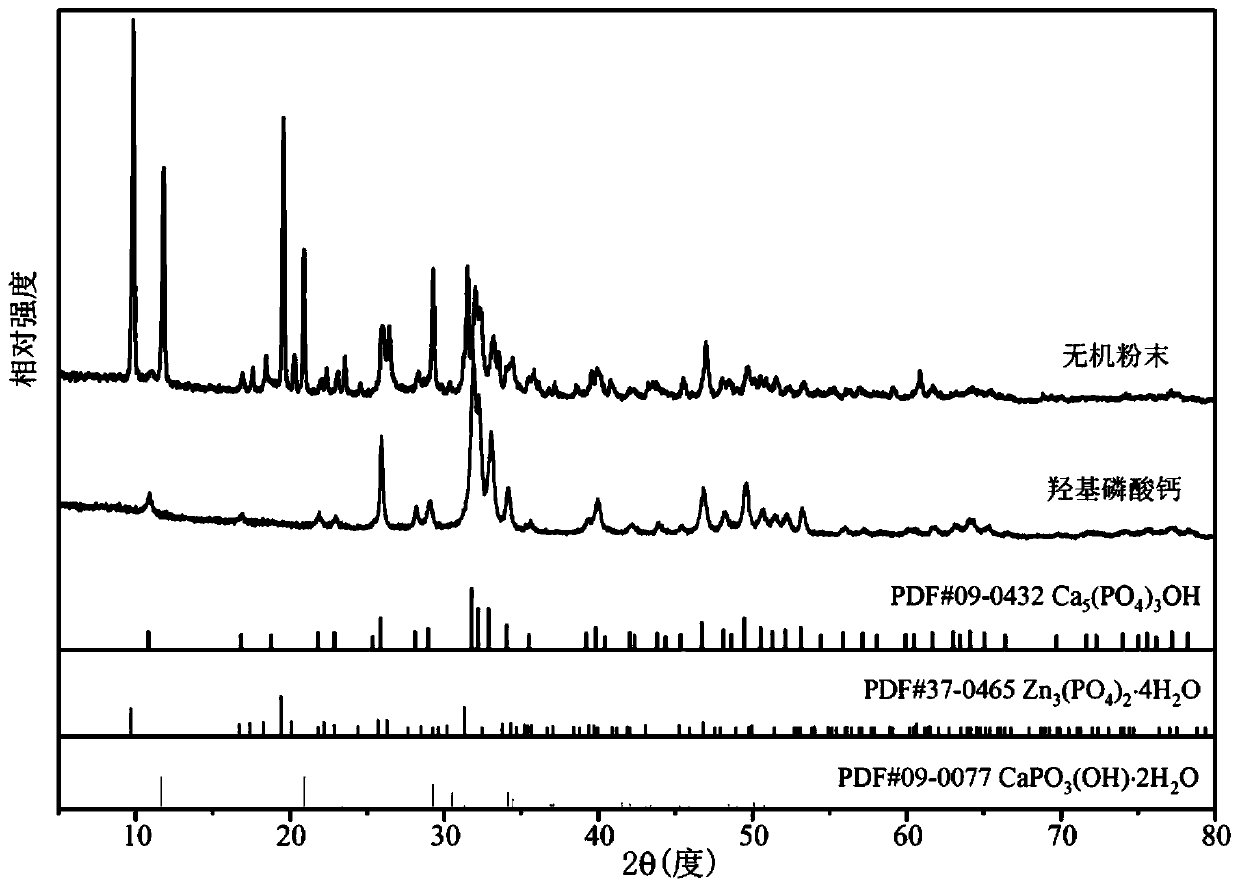
[0039] The inorganic powder and calcium hydroxyphosphate prepared in this example were characterized by an X-ray diffra...
Embodiment 2
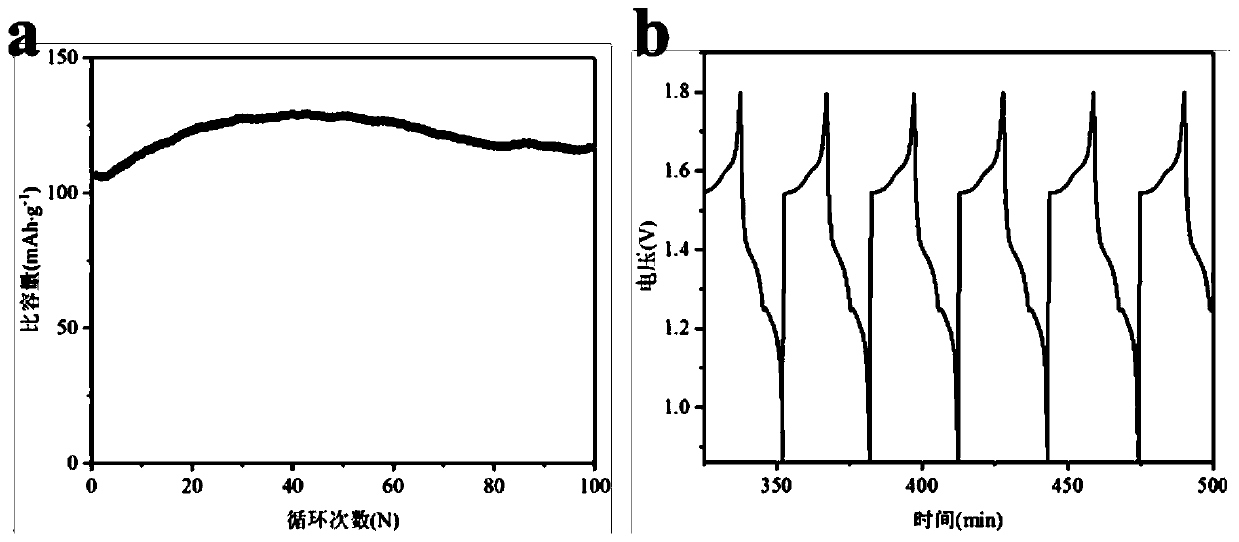
[0044] The colloidal electrolyte preparation method of this embodiment is basically the same as that of Example 1, but the thickness of the colloidal electrolyte used to assemble the zinc-ion battery is 0.8mm, and the CR2016 button battery is assembled together with the manganese dioxide positive electrode and the zinc sheet negative electrode, at 0.5A g -1 Under the current density of 0.85 ~ 1.8V voltage range, use the LAND test system to measure its electrochemical performance, get Figure 6 The cycle performance diagram and partial charge-discharge curve diagram.
[0045] Figure 6 a is the cycle performance diagram of the zinc-ion battery assembled by the colloidal electrolyte of this embodiment, and the initial specific volume is 163mA h g -1 , the specific capacity after 100 cycles is 164mA h g -1 , the curve is basically level, compared with Example 1, it shows that increasing the thickness of the colloidal electrolyte can effectively improve the cycle stability of th...
Embodiment 3
[0047] Solution configuration: ① Dissolve zinc sulfate in deionized solution to prepare 2mol / L zinc sulfate; ② Dissolve zinc sulfate and manganese sulfate in deionized water to prepare 2mol / L zinc sulfate + 0.1mol / L manganese sulfate liquid electrolyte.
[0048] Add calcium hydroxyphosphate into 2mol / L zinc sulfate solution at a mass ratio of 3:10. After mixing, ultrasonically treat for 2 hours to allow sufficient calcium-zinc ion exchange reaction to occur, and then carry out suction filtration. Drying for 12 hours under the hood to obtain inorganic powder;
[0049] Grind the inorganic powder into a fine powder in a mortar, add it into the liquid electrolyte according to the fact that the inorganic powder accounts for 60% of the mass of the electrolyte, mix evenly, and press it into a sheet with a thickness of 0.5 mm to obtain the inorganic colloidal electrolyte.
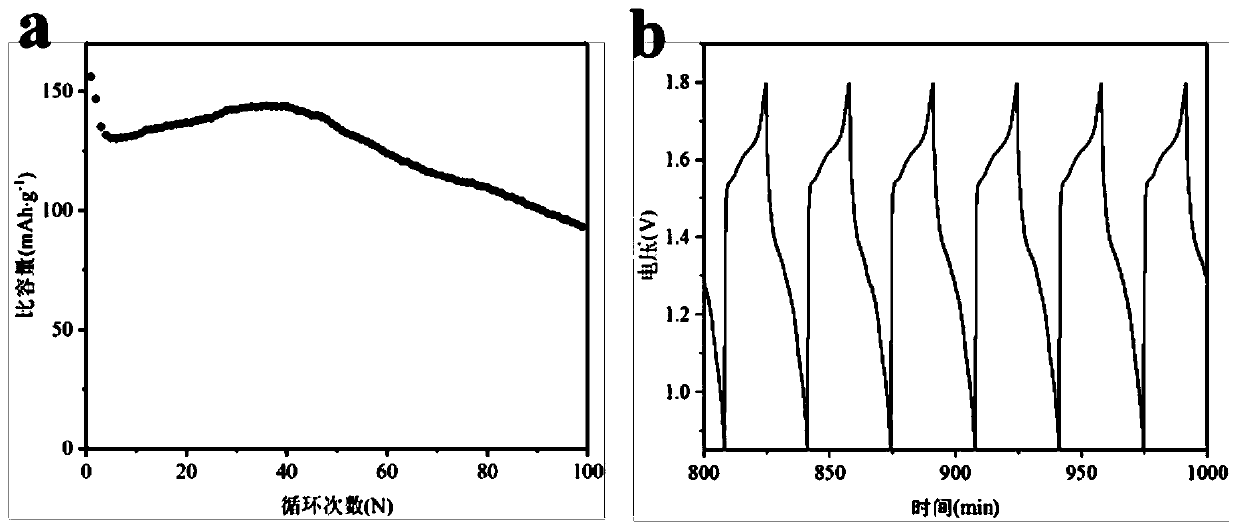
[0050] Using the colloidal electrolyte prepared in this example, the positive electrode of manganese dioxide, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com