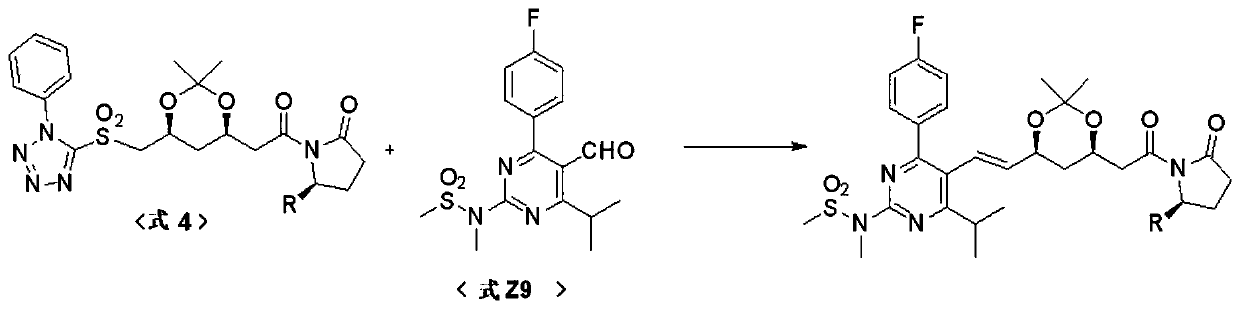
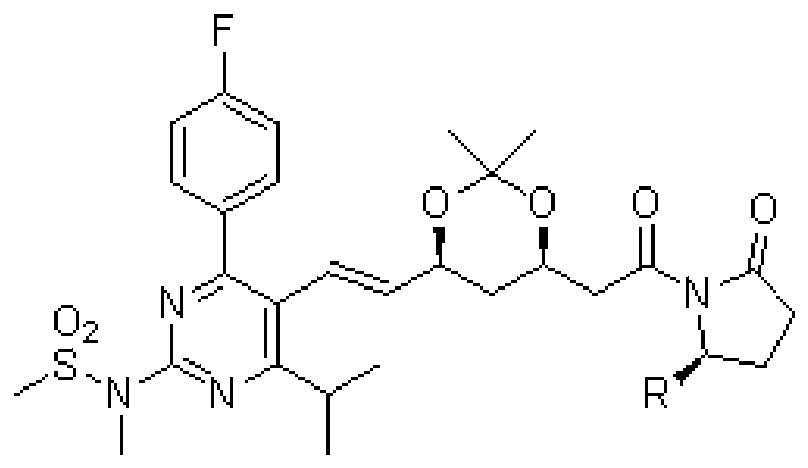
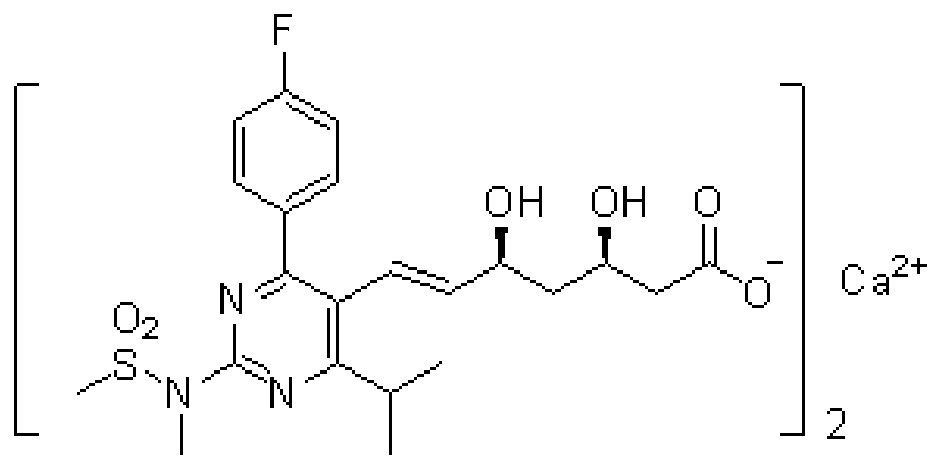
Preparation method of statin compounds and intermediates thereof
A compound and statin technology, applied in the field of preparation of statin compounds and their intermediates, can solve the problems of complex synthesis routes, difficult quality control and purification of oily substances, and difficulty in obtaining product purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Preparation of compound 2
[0086] At room temperature, PMTA (3.2g, 18.0mmol) was dissolved in DMF (20mL), and Na 2 CO 3 (2.2g, 21.6mmol) and compound 1 (4g, 18mmol), heated to 90°C, reacted for 24h, detected by LC-Ms.
[0087] After adding 10 mL of water, adjust the pH=5-6 with 10% HCl, extract with dichloromethane (40 mL×2), combine the organic phases with water (10 mL×2), dry, and concentrate in vacuo at 40° C. to obtain an oil, which is washed with in the next step.
[0088] LC-Ms: [M+H] = 365.20.
[0089] Preparation of compound 3
[0090] At room temperature, add iPrOH (20mL) to the oil in the previous step, then add ammonium heptamolybdate (1.1g) and H 2 o 2 (20 mL), after the addition was completed, the stirring reaction was continued at 25° C. for 18 h, and the reaction was complete as detected by Lc-Ms.
[0091] After adding 60 mL of dichloromethane for extraction and separation, the hydrogen peroxide was quenched with saturated sodium bisulfite, the li...
Embodiment 2
[0107] Preparation of Compound 2
[0108] At room temperature, PMTA (12.0g, 67.5mmol) was dissolved in DMF (50mL), and Na 2 CO 3 (7.1g, 67.5mmol) and compound 1 (10g, 45.0mmol), heated to 90°C, and reacted for 24h to complete the reaction.
[0109] After adding 25 mL of water, adjust the pH=5-6 with 10% HCl, extract with dichloromethane (100 mL×2), combine the organic phases with water (25 mL×2), dry, and concentrate in vacuo at 40° C. to obtain an oil, which is washed with in the next step.
[0110] Preparation of compound 3
[0111] At room temperature, add iPrOH (50mL) to the oil in the previous step, then add ammonium heptamolybdate (2.7g) and H 2 o 2 (50mL), after the addition was completed, the reaction was continued to stir at 25°C for 18h and the reaction was complete.
[0112] After adding 150 mL of dichloromethane for extraction and separation, the hydrogen peroxide was quenched with saturated sodium bisulfite, the liquid was separated, and the organic phase wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com