5-(2,5-bis(4-chloro-2-isopropylphenyl)thiophen-3-yl)-1h-tetrazole for the topical treatment of bacterial infections
A technology of cumene and thiophene, which can be used as a dermatologically acceptable carrier to eliminate pathogenic bacteria in diabetic ulcers, in the field of cellulitis drugs, and can solve problems such as multidrug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 - Synthesis of 5-(2,5-bis(4-chloro-2-isopropylphenyl)thiophen-3-yl)-1H-tetrazole
[0067] Refer to the following chemical schemes:
[0068]
[0069] Bromonitrile 2 (1.0 g, 3.7 mmol), isopropylphenyl borate 1 (2.31 g, 8.2 mmol, 2.2 equivalents) and Pd (PPh 3 ) 4 (0.22 g, 0.19 mmol, 0.05 equiv) was added to a round bottom flask and vacuum was applied for 3 seconds and flushed with argon 3 times. To this mixture was added 2M aqueous sodium carbonate solution (9.4ml, 18.8mmol, 5eq) and 20ml of dioxane. The reaction was stirred overnight at 95 °C, then quenched with water. The dark mixture was extracted with ethyl acetate (2 x 20ml), dried over anhydrous sodium sulfate, filtered and concentrated by rotary evaporation. Thiobenzonitrile 3 (1.4 g, 90%) was obtained by flash column chromatography (25% DCM in hexane).
[0070] Thiobenzonitrile 3 (1.0g, 2.4mmol), sodium azide (0.39g, 6.0mmol, 2.5eq) and zinc bromide (1.36g, 6.0mmol, 2.5eq) were mixed with 20ml of...
Embodiment 2
[0072] Example 2 (prophetic)
[0073] Azide-free Synthesis of 5-(2,5-bis(4-chloro-2-isopropylphenyl)thiophen-3-yl)-1H-tetrazole
[0074] 5-(2,5-Bis(4-chloro-2-isopropylphenyl)thiophen-3-yl)-1H-tetrazole can also be prepared by protecting the tetrazole to give a mixture of N-protected tetrazoles . This step is followed by bromination to give the N-protected 5-bromotetrazole mixture, followed by palladium cross-coupling using thiophen-3-ylboronic acid. The resulting product was then brominated at the 2- and 5-positions of thiophene, followed by palladium cross-coupling using (4-chloro-2-isopropylphenyl)boronic acid. Deprotection of the N-protected tetrazole moiety decomposes the mixture into single products. Deprotection may be combined with or subsequently introduced into a pharmaceutically acceptable salt moiety using methods known in the art. A possible synthesis is given in the following scheme, where the nitrogen protecting group is benzyl:
[0075]
[0076]
Embodiment 3-
[0078] Preparation of a pharmaceutical composition comprising 5-(2,5-bis(4-chloro-2-isopropylphenyl)thiophen-3-yl)-1H-tetrazole and a dermatologically acceptable carrier
[0079] Compounds were formulated by dissolving 50 mg of compound in 50 μl of 100% DMSO, then gradually added and mixed into 5 ml of Glaxal matrix (WellSpring Pharmaceutical Canada Corp., Oakville, Canada) at a final concentration of 1% (w / v) compound and 1% (v / v) DMSO.
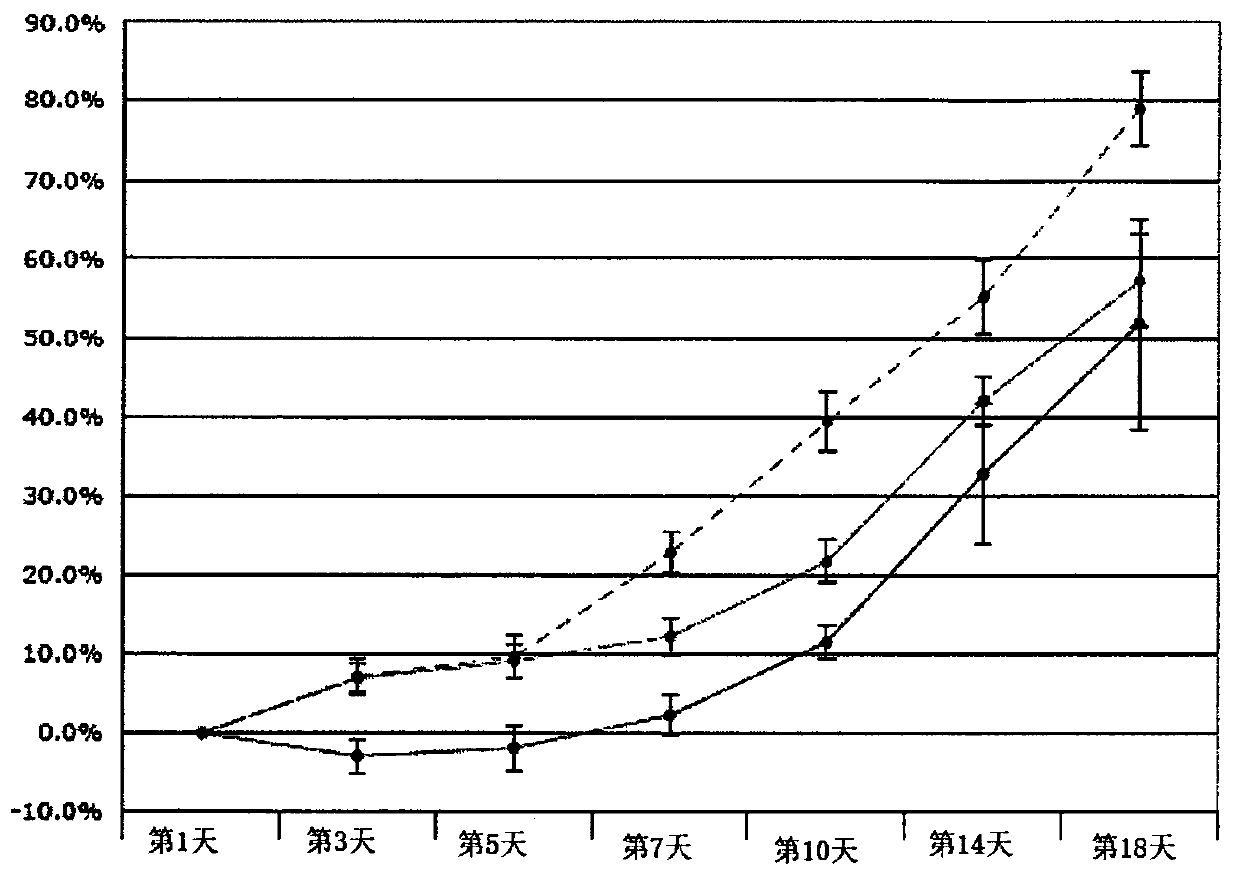
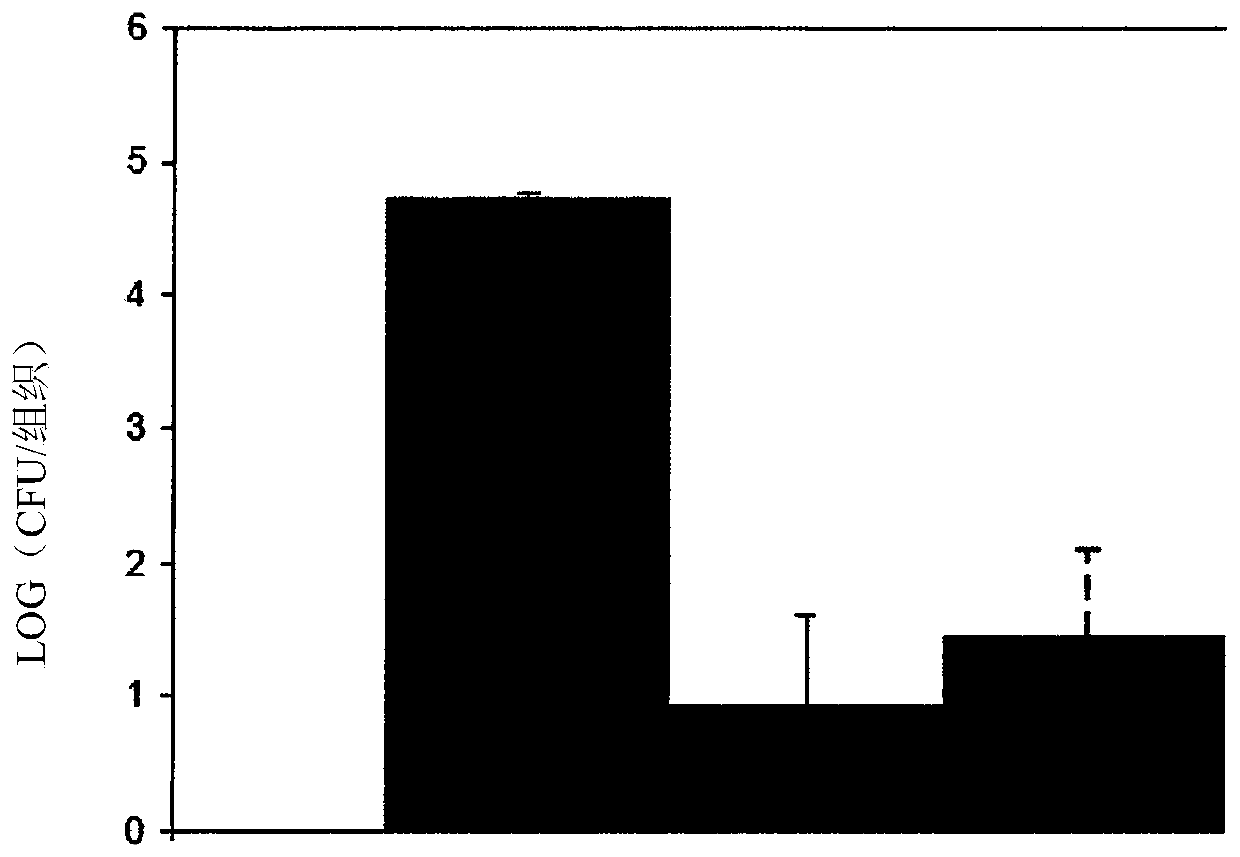
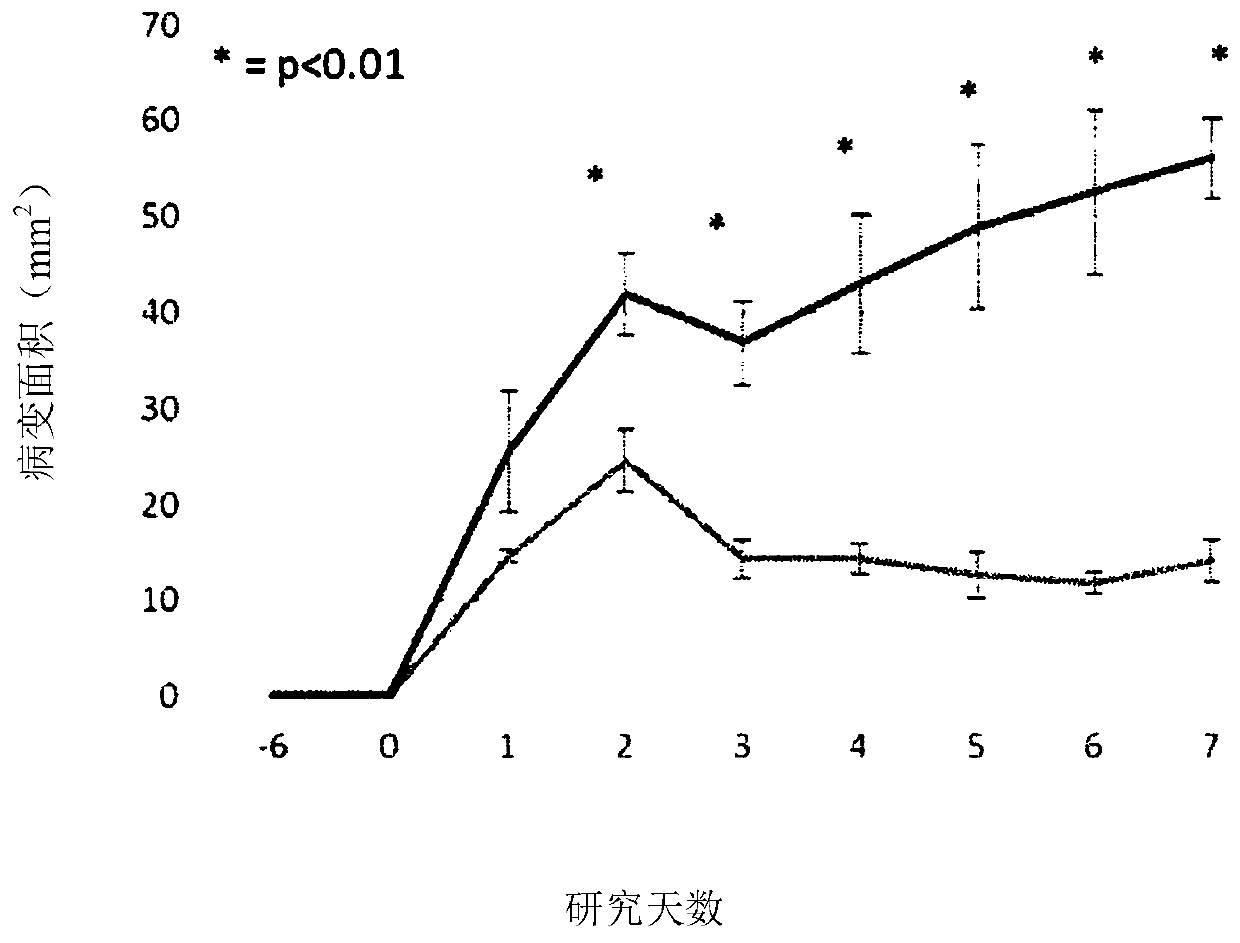
[0080] biological data
[0081] Minimum Inhibitory Concentration (MIC)
[0082] Using a broth microdilution assay according to Clinical Laboratory Standards Institute (CSLI) guidelines, we tested 5-(2,5-bis(4-chloro-2-isopropylphenyl)thiophen-3-yl)- 1H-tetrazole ("test sample" in the table below), established MICs against a variety of MDR bacteria and indicated associations with methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci ( VRE) and substantial activity of Streptococcus species. Antimicrobial susc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mic value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com