Preparation method of polysubstituted oxazole-2 (3H)-ketone compound
A ketone compound, multi-substitution technology, applied in the field of preparation of multi-substituted oxazol-2-one compounds, can solve problems such as limiting the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
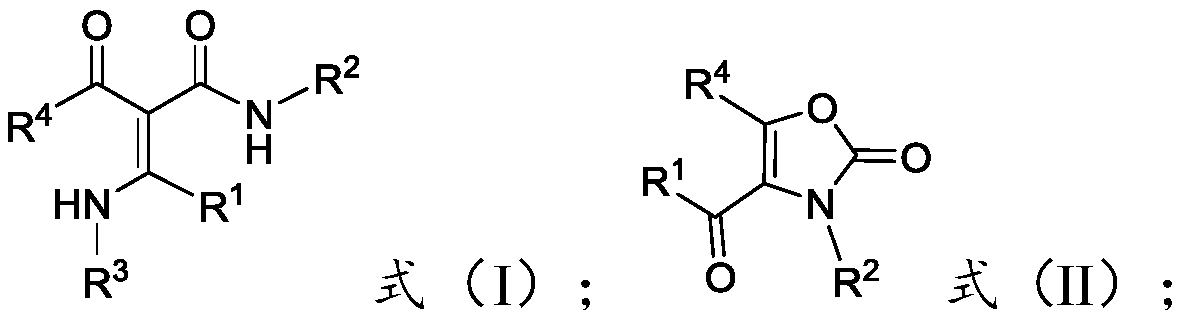
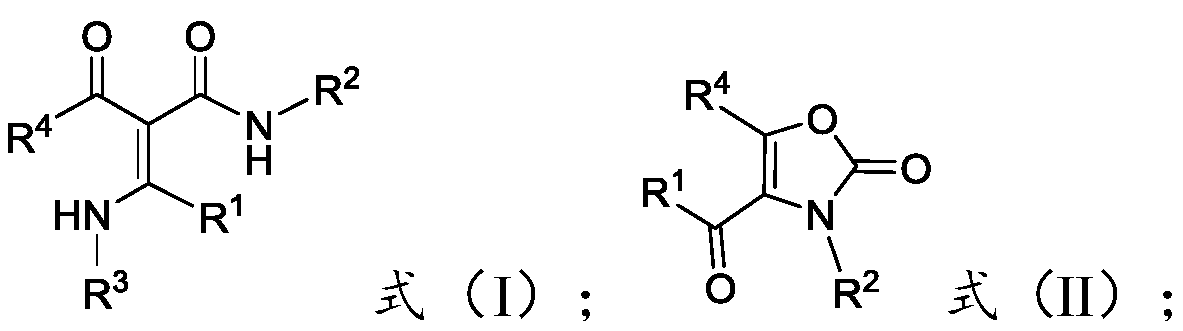
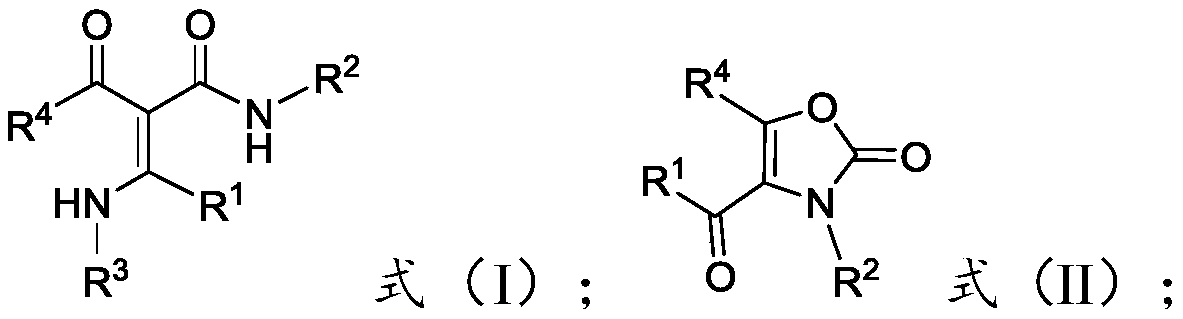
[0034] The present invention provides a preparation method of multi-substituted oxazol-2(3H)-one compounds, comprising: reacting acrylamide compounds represented by formula (I) with hypervalent iodine reagents in the presence of auxiliary agents, Obtain the multi-substituted oxazol-2(3H)-one compound represented by formula (II);
[0035]
[0036] Among them, -R 1 ,-R 2 with -R 4 Each independently is -A, -Ar or -Het; -R 3 for-A;
[0037] The -A is C1-C30 chain alkyl, C1-C30 substituted chain alkyl or C3-C7 cycloalkyl, preferably C1-C20 chain alkyl, C1-C20 substituted chain Alkyl or C3-C7 cycloalkyl, more preferably C1-C10 chain alkyl, C1-C10 substituted chain alkyl or C3-C7 cycloalkyl, more preferably C1-C5 chain Alkyl, C1~C5 substituted chain alkyl or C3~C7 cycloalkyl, most preferably C1~C3 chain alkyl, C1~C3 substituted chain alkyl or C3~C7 cycloalkyl .
[0038] The number of substituents in the C1-C30 substituted chain alkyl group is preferably 1-20, more preferab...
Embodiment 1
[0056] Synthesis of 3-(4'-methylphenyl)-4-acetyl-5-methyl-oxazol-2(3H)-one
[0057] At room temperature, add 1.5mmol of PIFA, 1.0mmol of TFA and 40.0mL of dichloromethane into a 50mL round bottom flask and mix well, then add 1.0mmol of N-(4′-methylphenyl)- 2-Acetyl-3-methylamino-2-butenamide, react at room temperature for 0.5 hours. After the reaction, pour the reaction solution into 50.0 mL of saturated NaCl aqueous solution, then extract three times with 20.0 mL of dichloromethane, combine the organic phases, add 3.0 g of anhydrous sodium sulfate to dry, filter to remove the solid, and then remove the organic solvent , the residue was separated by silica gel column chromatography (petroleum ether / ethyl acetate=5:1) to obtain 3-(4'-methylphenyl)-4-acetyl-5-methyl-oxazole-2( 3H)-ketone, 65% yield, >99% purity.
[0058] The 3-(4′-methylphenyl)-4-acetyl group-5-methyl-oxazol-2(3H)-one obtained in Example 1 is analyzed by nuclear magnetic resonance spectrum, and its hydrogen nu...
Embodiment 2
[0062] Synthesis of 3-(4'-methylphenyl)-4-acetyl-5-methyl-oxazol-2(3H)-one
[0063] At room temperature, add 2.0mmol of PhIO, 3.0mmol of TFA and 20.0mL of 1,2-dichloroethane into a 50mL round bottom flask and mix well, then add 1.0mmol of N-(4′-formazan phenyl)-2-acetyl-3-methylamino-2-butenamide, reacted at room temperature for 4.0 hours. After the reaction, pour the reaction solution into 50.0 mL of saturated NaCl aqueous solution, then extract three times with 20.0 mL of dichloromethane, combine the organic phases, add 3.0 g of anhydrous sodium sulfate to dry, filter to remove the solid, and then remove the organic solvent , the residue was separated by silica gel column chromatography (petroleum ether / ethyl acetate=5:1) to obtain 3-(4'-methylphenyl)-4-acetyl-5-methyl-oxazole-2( 3H)-ketone, 55% yield, >99% purity.
[0064] The 3-(4′-methylphenyl)-4-acetyl-5-methyl-oxazol-2(3H)-one obtained in Example 2 was analyzed by nuclear magnetic resonance spectrum, which was the sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com