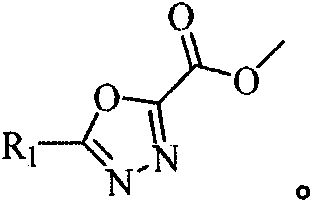
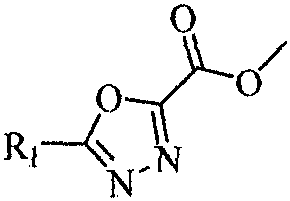
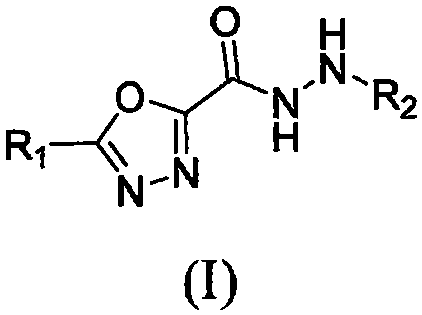
1, 3, 4-oxadiazole hydrazide compound as well as preparation method and application thereof
A technology of oxadiazole hydrazide and compounds, applied in the field of medicinal chemistry, can solve problems such as increased resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the preparation of methyl 2-chlorobenzoate
[0056] Add 10.0g (63.87mmol) 2-chlorobenzoic acid and 100 mL of anhydrous methanol into a 250mL round bottom flask, then add 4.0mL of concentrated sulfuric acid dropwise to the above mixed system, heat to reflux at about 78°C, and after 8h The reaction process was monitored by TLC, and the reaction of the raw materials was complete; the remaining methanol was distilled off under reduced pressure, then 50 mL of water was added to the system, extracted with ethyl acetate (150 mL×3), the organic phases were collected and combined, and dried by adding an appropriate amount of anhydrous sodium sulfate. Suction filtration, precipitation of the filtrate, weighing 11.3 g of yellow oily liquid, yield 94.5%.
Embodiment 2
[0057] Embodiment 2: the preparation of 2-chlorobenzohydrazide
[0058] In a 150mL round bottom flask, add 10.0g (58.62mmol) methyl 2-chlorobenzoate, then add 17.4mL (18.0g, 293.10mmol) 80% hydrazine hydrate, return to room temperature after 30 min of reaction under ice-bath conditions, After 2 hours, TLC monitors the reaction. The reaction of the raw materials is complete. Stop the reaction. A white solid can be seen. Suction filtration. The filter cake is washed 2-3 times with ice water, each time with about 5 mL of water. The filter cake is dried under an infrared lamp and weighed. 9.6 g of white solid powder of intermediate 2-chlorobenzohydrazide was obtained, and the yield was 96.0%.
Embodiment 3
[0059] Example 3: Preparation of 2-(2-(2-chlorobenzoyl)hydrazino)-2-oxoacetic acid methyl ester
[0060] Take 12.0g 2-chlorobenzohydrazide in a 500mL round bottom flask, add 235mL DCM and stir to dissolve; add 10.0mL Et 3 N, after 15 minutes, transfer to ice bath; mix 30mL DCM with 7.76mL monomethyl oxalyl chloride, and add dropwise to the above system with a constant pressure dropping funnel; remove the ice bath after the dropwise addition, and return to room temperature; After 10 h, the reaction progress was monitored by TLC, and the reaction was completed. Add 50 mL of water to dissolve the salt in the system after distillation under reduced pressure, extract with EA (100 mL×5); combine the organic phases, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain 17.3 g of light yellow solid compound, the crude yield is 95.8 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com