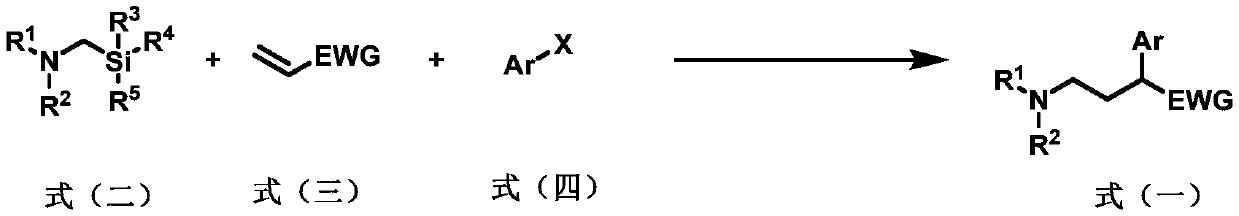
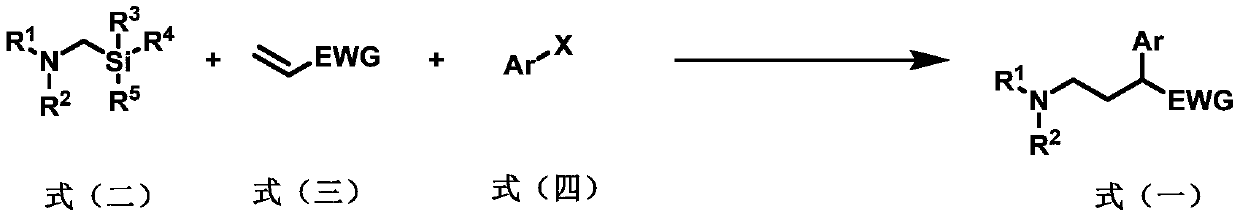
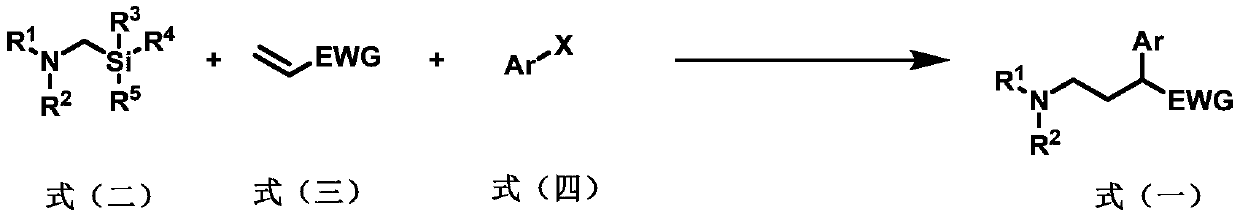
Preparation method of 2-aryl-gamma-aminobutyric acid derivative
An aminobutyric acid and derivative technology, which is applied in the field of preparation of 2-aryl-γ-aminobutyric acid derivatives, can solve the problems of poor compatibility of reaction functional groups, cumbersome steps, harsh reaction conditions, etc., and achieves the universality of substrates. Wide range of properties, good reaction selectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Add a magnetic stirrer in a 10mL transparent glass reaction tube, photocatalyst Ir(ppy) 2 (bpy)PF 6 (2.4mg, 3.0μmol, 0.010eq), metal catalyst NiCl 2 Glyme (6.6mg, 30μmol, 0.10equivalent), ligand di(OMe)bpy (6.1mg, 30μmol, 0.10equivalent), seal the nozzle with a rubber stopper, protect the reaction system with nitrogen gas (exhaust three times), inject the syringe into Solvent anhydrous DMF (3.0 mL), followed by microsyringe addition of α-aminomethylsilyl 1a (0.12 mL, 0.10 g, 0.60 mmol, 2.0 eq), benzyl acrylate 2a (90 μL, 97 mg, 0.60 mmol, 2.0 eq) , iodobenzene 3a (33μL, 61mg, 0.30mmol, 1.0eq), reacted at room temperature under blue light for 15 hours, then quenched the reaction with water, extracted with ethyl acetate (20mL x 3 times), combined the organic phases, and anhydrous sulfuric acid Sodium-dried, filtered, spin-dried, and column chromatography gave product 4 (78 mg, yield 77%). 1 H NMR (400MHz, CDCl 3)δ:7.34–7.22(m,10H),5.13(d,J=12.5Hz,1H),5.07(d,J=12.5Hz,1...
Embodiment 2
[0062] Add a magnetic stirrer in a 10mL transparent glass reaction tube, photocatalyst Ir(ppy) 2 (bpy)PF 6 (2.4mg, 3.0μmol, 0.010eq), metal catalyst NiCl 2 Glyme (6.6mg, 30μmol, 0.10equivalent), ligand di(OMe)bpy (6.1mg, 30μmol, 0.10equivalent), seal the nozzle with a rubber stopper, protect the reaction system with nitrogen gas (exhaust three times), inject the syringe into Solvent Anhydrous DMF (3.0 mL), followed by microsyringe addition of α-aminomethylsilyl 1b (0.10 g, 0.60 mmol, 0.60 mmol, 2.0 equiv), benzyl acrylate 2a (90 μL, 97 mg, 0.60 mmol, 2.0 equiv) , iodobenzene 3a (33μL, 61mg, 0.30mmol, 1.0eq), reacted at room temperature under blue light for 15 hours, then quenched the reaction with water, extracted with ethyl acetate (20mL x 3 times), combined the organic phases, and anhydrous sulfuric acid Sodium-dried, filtered, spin-dried, and column chromatography gave product 5 (90 mg, yield 88%). 1 H NMR (400MHz, CDCl 3 )δ:7.37–7.22(m,10H),5.14(d,J=12.4Hz,1H),5.06(d,J...
Embodiment 3
[0064] Add a magnetic stirrer in a 10mL transparent glass reaction tube, photocatalyst Ir(ppy) 2 (bpy)PF 6 (2.4mg, 3.0μmol, 0.010eq), metal catalyst NiCl 2 Glyme (6.6mg, 30μmol, 0.10equivalent), ligand di(OMe)bpy (6.1mg, 30μmol, 0.10equivalent), seal the nozzle with a rubber stopper, protect the reaction system with nitrogen gas (exhaust three times), inject the syringe into Solvent anhydrous DMF (3.0 mL), followed by addition of α-aminomethylsilyl 1c (0.11 g, 0.60 mmol, 0.60 mmol, 2.0 equiv), benzyl acrylate 2a (90 μL, 97 mg, 0.60 mmol, 2.0 equiv) with a micro syringe , iodobenzene 3a (33μL, 61mg, 0.30mmol, 1.0eq), reacted at room temperature under blue light for 15 hours, then quenched the reaction with water, extracted with ethyl acetate (20mL x 3 times), combined the organic phases, and anhydrous sulfuric acid Sodium-dried, filtered, spin-dried, and column chromatography gave product 6 (95 mg, yield 90%). 1 H NMR (400MHz, CDCl 3 )δ:7.34–7.27(m,8H),7.26–7.22(m,2H),5.14(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com