A kind of method for densification of barrier layer of solid oxide battery
A solid oxide and barrier layer technology, applied in fuel cells, battery electrodes, circuits, etc., can solve problems such as cumbersome processes, achieve the effects of avoiding mutual reactions, reducing calcination temperature, and improving battery performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
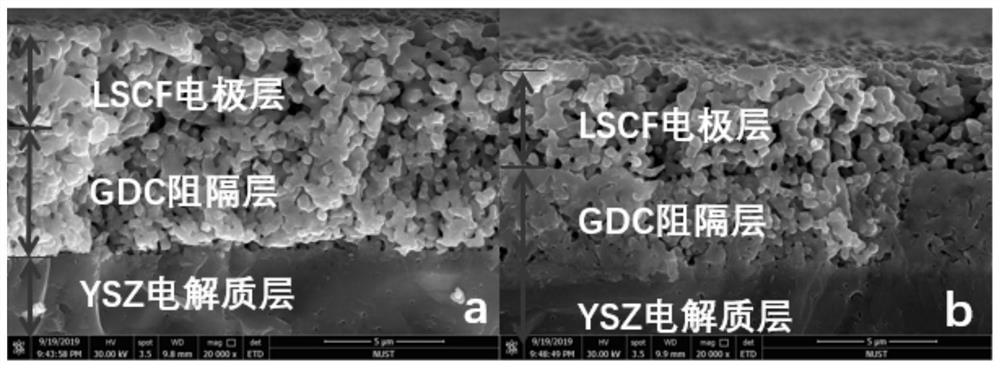
[0017] Differences in densification of battery barrier layers with different solute ratios
[0018] First, the upper isolation layer GDC was screen-printed on the dense YSZ electrolyte sheet, and then calcined at 1250 °C for 3 h in a muffle furnace.
[0019] Then configure two parts of hydrothermal solution, add 60ml deionized water in 100ml reactor, and then add Ge(NO 3 ) 3 ·6H 2 O and Ce(NO 3 ) 3 ·6H 2 O in a molar ratio of 0.1:0.9 (Ce 0.9 Gd 0.1 o 2-m ) solution, that is, the nitric acid hydrate solution crystal with the same composition as GDC; the second hydrothermal solution Ge(NO 3 ) 3 ·6H 2 O and Ce(NO 3 ) 3 ·6H 2 O at a molar ratio of 0.1:1 (CeGd 0.1 o 2-m ) solution. The cells were reacted in two hydrothermal solutions for 24 hours.
[0020] Then soak the electrolyte sheet with GDC screen printed on it in alcohol for ten minutes, and then wipe it clean. The electrolyte sheet was put into a reaction kettle added with a hydrothermal solution, and the ...
Embodiment 2
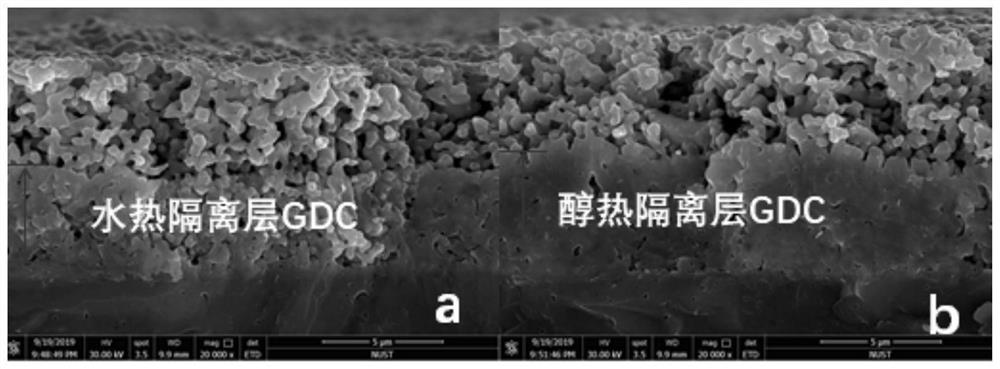
[0024] Effect of different hydrothermal solvents on battery densification
[0025] Ge(NO 3 ) 3 ·6H 2 O and Ce(NO 3 ) 3 ·6H 2 O in a molar ratio of 0.1:0.9 (Ce 0.9 Gd 0.1 o 2-m ), using deionized water and absolute ethanol as solvents respectively, that is, nitric acid hydrate solution crystals with the same composition as GDC. The electrolyte sheet was put into a reaction kettle added with a hydrothermal solution, and the reaction kettle was placed in an oven and reacted at 180°C for 24h.
[0026] Finally, the electrolyte sheet was taken out, and LSCF was screen-printed on the GDC of the electrolyte sheet with an area of 0.5 cm, and calcined at 1075° C. for 2 h. Then add silver mesh and silver wire current collector on both sides to make a symmetrical battery.
[0027] The end result is as image 3 It can be seen that the battery with ethanol as the solvent has a higher density of the isolation layer, and the pores of the alcohol thermal reaction are smaller than ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com