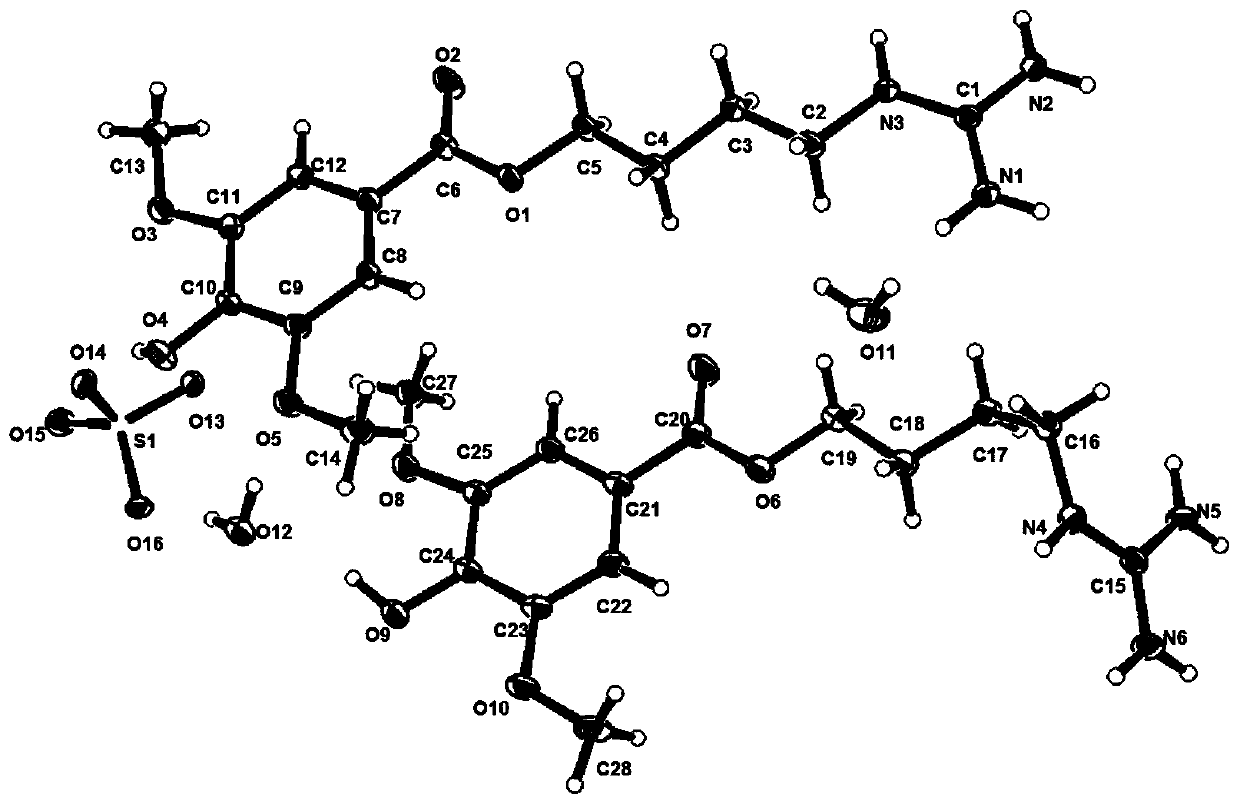
Application of leonurine and crystals thereof in preparation of medicine for resisting hyperhomocysteinemia
A technology of leonurine and application, which is applied in the field of preparing anti-hyperhomocysteinemia drugs to achieve the effect of reducing the incidence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 In ApoE - / - To verify the effects of motherwortine and its A-type crystals on blood Hcy levels in the animal model of metabolic disorders fed with high-fat feed
[0048] Experimental methods include: ApoE - / - The mice were fed with normal feed in the SPF grade animal room until 6 weeks old, and then randomly divided into 5 groups according to the following grouping scheme for feeding and administration:
[0049] 1) Control group (ApoE - / - Mice were fed with normal feed, n=14), and triple-distilled water was administered orally (1 ml / kg body weight) once a day;
[0050] 2) Model solvent control group (ApoE - / - Mice, high-fat diet, n=16), triple distilled water gavage (1 ml / kg body weight) once a day;
[0051] 3) Leonurine A crystal low dose group (ApoE - / - Mice, high-fat feed, n=8), and once a day, the motherwortine type A crystal solution was administered orally (the drug dose was 10 mg / kg / day, and the drug concentration was 10 mg / ml);
[0052] 4) Medium...
Embodiment 2
[0057] Example 2 Detection of mouse plasma Hcy level by enzyme-linked immunosorbent assay
[0058] Experimental principle: The kit uses a competitive method to detect the content of Hcy in the sample; add the sample to the antibody-coated enzyme-labeled well, then add the biotin-labeled recognition antigen, and incubate at 37°C for 30 minutes. Antibodies compete for binding to form immune complexes, wash with PBST to remove unbound biotin antigen, then add avidin-HRP, incubate at 37°C for 30min, avidin-HRP binds to biotin antigen, and the bound HRP is washed Catalyze TMB (tetramethylbenzidine) into blue, and then turn into yellow under the action of acid, with an absorption peak at 450nm wavelength, and the absorbance value is negatively correlated with the concentration of antigen in the sample;
[0059] Operation steps: Please equilibrate the kit at room temperature for half an hour before use;
[0060] Blank well: no sample is added, only chromogen A, B and stop solution a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com