Recombinant beta-galactosidase and construction method and application thereof
A technology of galactosidase and construction method, applied in the field of recombinant β-galactosidase and its construction, can solve problems such as loss of enzyme activity, contamination by miscellaneous bacteria, difficulty in separation and purification, etc. The effect of broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Construction of recombinant expression plasmid
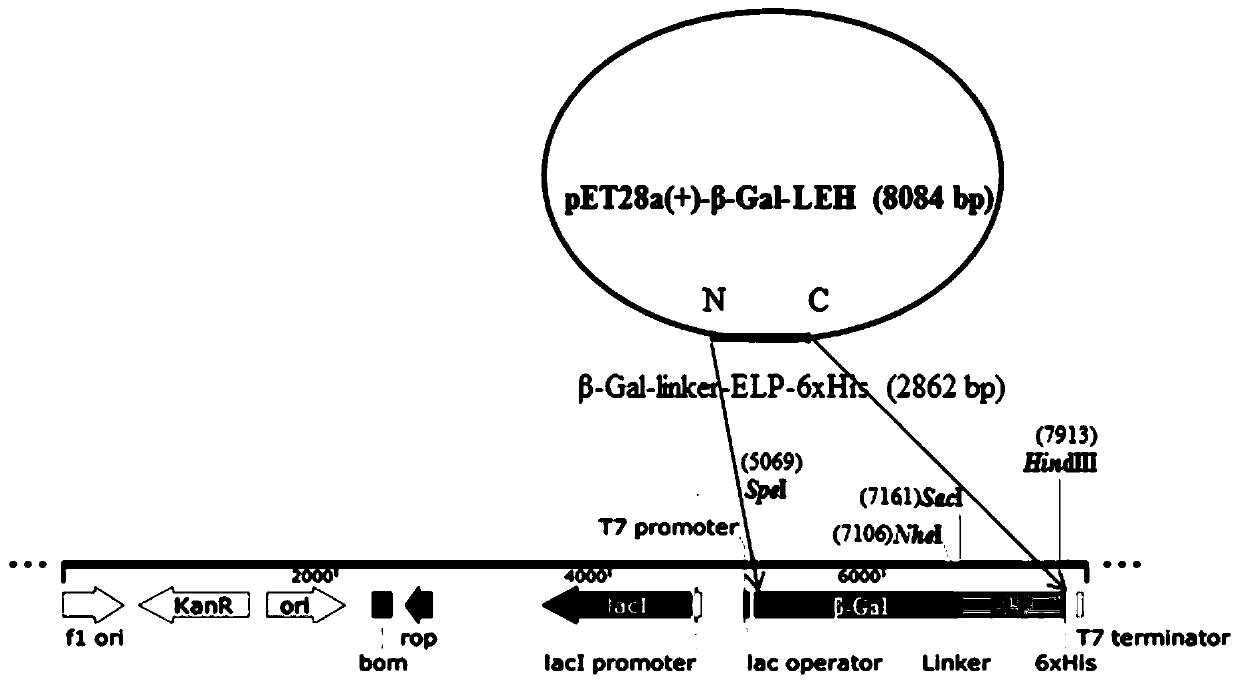
[0025] The β-Gal gene comes from ( Lactobacillus sp.B164, accession number JF345715.1), the β-Gal nucleotide sequence synthesized by the whole gene of the biological company, and the 5' and 3' were respectively equipped with Spe I and Nhe I, and the whole gene was synthesized with Spe I The β-Gal nucleotide sequence of the Nhe I restriction site was constructed on the pet28a (+) plasmid, named pet28a (+)-β-Gal.
[0026] Biological company synthesized the Linker-ELP nucleotide sequence from the whole gene, with Nhe I restriction site at the 5' of linker, Hind I restriction site at the 3' of ELP, and Sac I restriction site between Linker and ELP site. Then the above sequence was constructed on the pUC18 plasmid, named as pUC18-Linker-ELP plasmid.
[0027] Use Nhe I and Hind I to double-digest pet28a(+)-β-Gal and PUC18-Linker-ELP, and the double-digestion system is:
[0028] NheI: 1 μl;
[0029] Hind I: 1 ...
Embodiment 2
[0040] Example 2: Expanded culture of Escherichia coli expressing β-Gal-LEH
[0041] (1) Transfer freshly transformed recombinant Escherichia coli to solid LB medium supplemented with 50 μg / mL kanamycin (agar powder 15g / L, tryptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L, pH7.4), and cultured at 37 ℃, and then transfer the single colony after overnight culture to 5ml liquid LB medium containing 50 μg / mL kanamycin (tryptone 10g / L, Yeast extract 5g / L, sodium chloride 10g / L, pH 7.4), and cultured overnight at 37°C in an orbital shaker at 200 rpm, then inoculated 3 mL of overnight cultured bacteria into 300 mL containing 50 μg Grow the liquid LB medium of kanamycin / mL at 37 °C, 200 rpm for 3 hours or the OD600 of the bacteria reaches between 0.4-0.6.
[0042](2) Place the liquid medium finally obtained in step (1) on ice for 20 minutes, add isopropyl β-D-1-thiogalactopyranoside (IPTG) to it to a final concentration of 0.4 mM, Then shake culture at 180 rpm at 16°C, 25°C ...
Embodiment 3
[0045] Example 3: Purification of β-Gal-LEH by Reversible Phase Change Cycle (ITC)
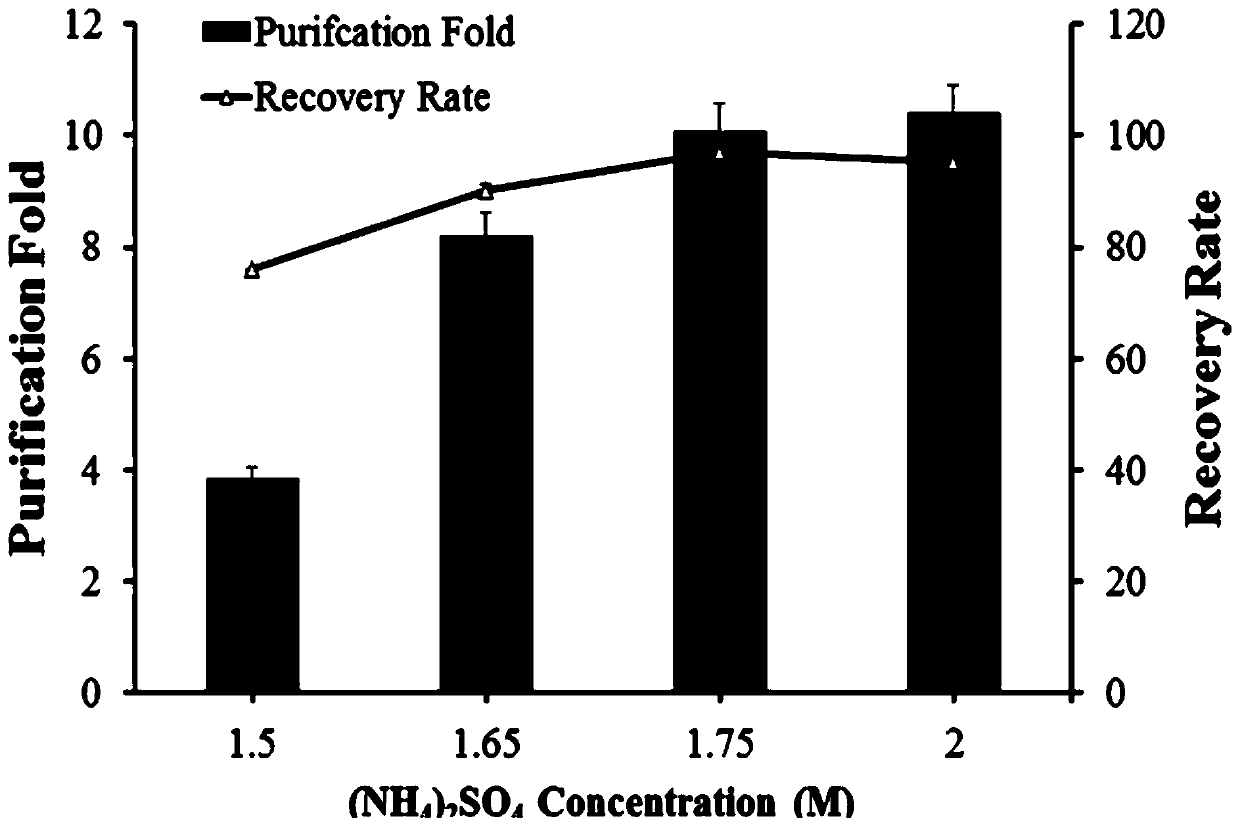
[0046] In this example, a reversible phase transition cycle was used to purify β-galactosidase, so as to verify the self-purification performance and purification efficiency of β-galactosidase prepared in Example 2. An appropriate concentration of 1.0-2.0 M (NH 4 ) 2 SO 4 Add to 500 μL of clarified sample lysate, then shake at 1000 rpm at 25 °C for 20 minutes, then spin the sample at 14,000 rpm at 25 °C for 30 minutes, discard the supernatant, and resuspend the pellet by pipetting extensively. Suspend in 1 mL of cold Tris-HCl buffer (50 mM, pH 8.0). The samples were then placed on ice for 60 minutes, followed by centrifugation at 13,000 rpm for 30 minutes at 4 °C to obtain the supernatant containing the purified protein of interest, which was transferred to a new EP tube. Save a portion of the purified sample for SDS-PAGE analysis.
[0047] figure 2 for different concentrations (NH 4 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com