Recombinant human growth hormone, and construction method and application thereof
A technology of human growth hormone and growth hormone, which is applied in the field of recombinant human growth hormone and its construction, can solve the problems of difficulty in prolonging the half-life of growth hormone, short half-life of natural growth hormone, and increasing the residence time of drugs to achieve efficient self-purification and efficient storage The effect of energy, high resilience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
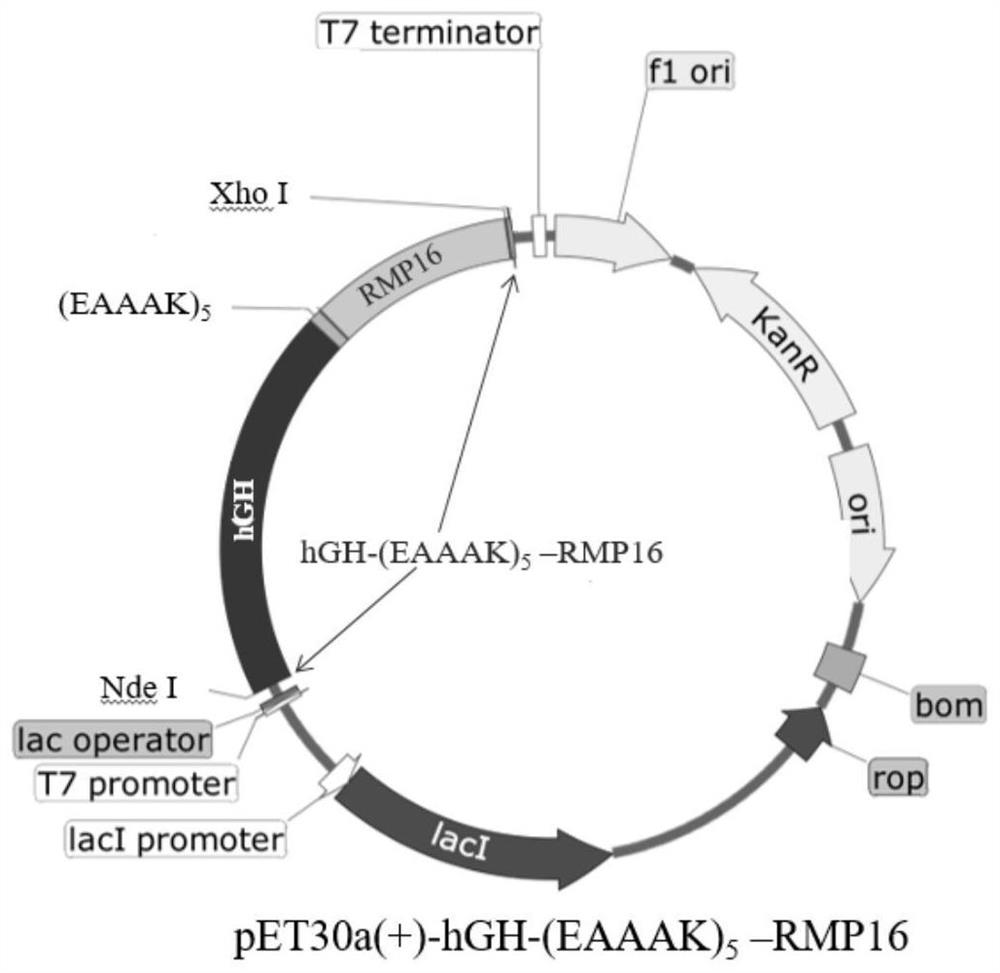
[0026] Example 1: Construction of recombinant expression plasmid pET30a(+)hGH-(EAAAK)5-RMP16
[0027] Sangon Bioengineering (Shanghai) Co., Ltd. Whole Gene Synthesis hGH-(EAAAK) 5 - RMP16, wherein, the nucleic acid sequence of hGH is shown in SEQ ID NO: 3; RMP16 is a resin-mimetic protein (resilin-mimetic protein), its amino acid sequence is shown in SEQ ID NO: 4, and the nucleotide sequence encoding RMP16 is shown in Shown in SEQ ID NO:5; linker (EAAAK) 5 is a connecting peptide, and its nucleic acid sequence is shown in SEQ ID NO:6.
[0028] SEQ ID NO: 3:
[0029]ATGGCTACAGGCTCCCGGACGTCCCTGCTCCTGGCTTTTGGCCTGCTCTGCCTGCCCTGGCTTCAAGAGGGCAGTGCCTTCCCAACCATTCCCTTATCCAGGCTTTTTGACAACGCTATGCTCCGCGCCCATCGTCTGCACCAGCTGGCCTTTGACACCTACCAGGAGTTTGAAGAAGCCTATATCCCAAAGGAACAGAAGTATTCATTCCTGCAGAACCCCCAGACCTCCCTCTGTTTCTCAGAGTCTATTCCGACACCCTCCAACAGGGAGGAAACACAACAGAAATCCAACCTAGAGCTGCTCCGCATCTCCCTGCTGCTCATCCAGTCGTGGCTGGAGCCCGTGCAGTTCCTCAGGAGTGTCTTCGCCAACAGCCTGGTGTACGGCGCCTCTGACAGCAACGTCTATGACCTCC...
Embodiment 2
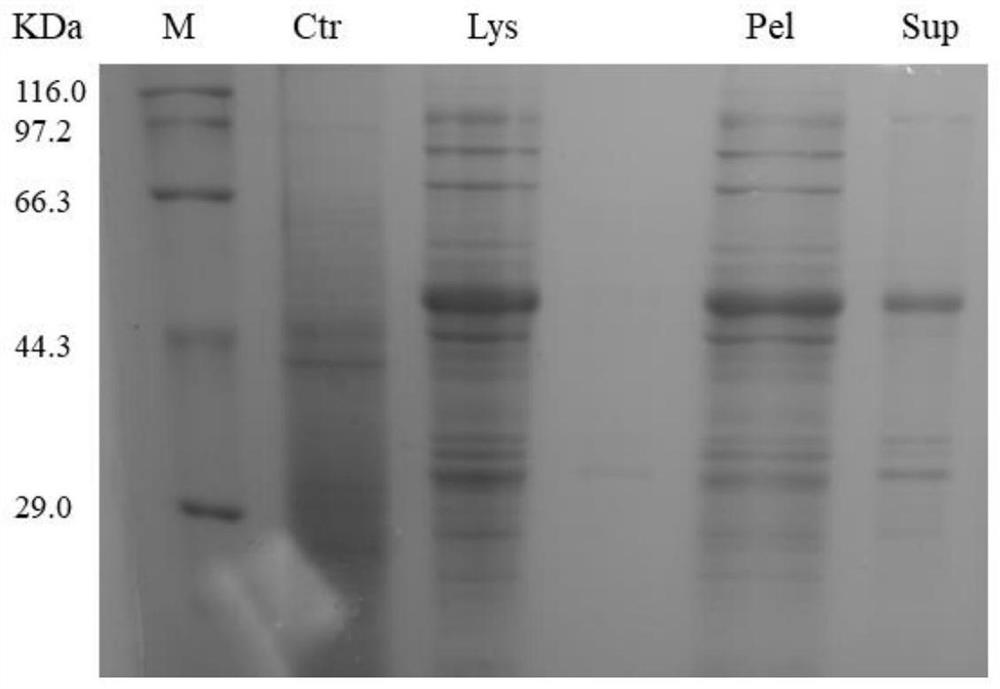
[0054] Embodiment 2: expand the escherichia coli expressing hGH-(EAAAK) 5-RMP16
[0055] (1) Transfer freshly transformed recombinant Escherichia coli BL21 to solid LB medium with 50 μg / mL kanamycin (agar powder 15g / L, tryptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L, pH7.4), cultivate overnight at 37 ℃, and then transfer the single colony after overnight culture to 5ml liquid LB medium containing 50 μg / mL kanamycin (tryptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L, pH 7.4), and cultivated overnight at 37 ℃, 00 rpm orbital shaker, then inoculated 3 mL of overnight cultured E. coli BL21 in 300 mL containing 50 Grow the liquid LB medium of μg / mL kanamycin at 37 °C, 200 rpm for 3 hours or the OD600 of the bacteria reaches between 0.4-0.6.
[0056] (2) Place the liquid LB medium containing recombinant E. coli finally obtained in step (1) on ice for 20 minutes, and add isopropyl β-D-1-thiogalactopyranoside (IPTG) to it to a final concentration of 0.2 mM. Then, ...
Embodiment 3
[0059] Embodiment 3: cooling coagulation purification hGH-(EAAAK) 5-RMP16
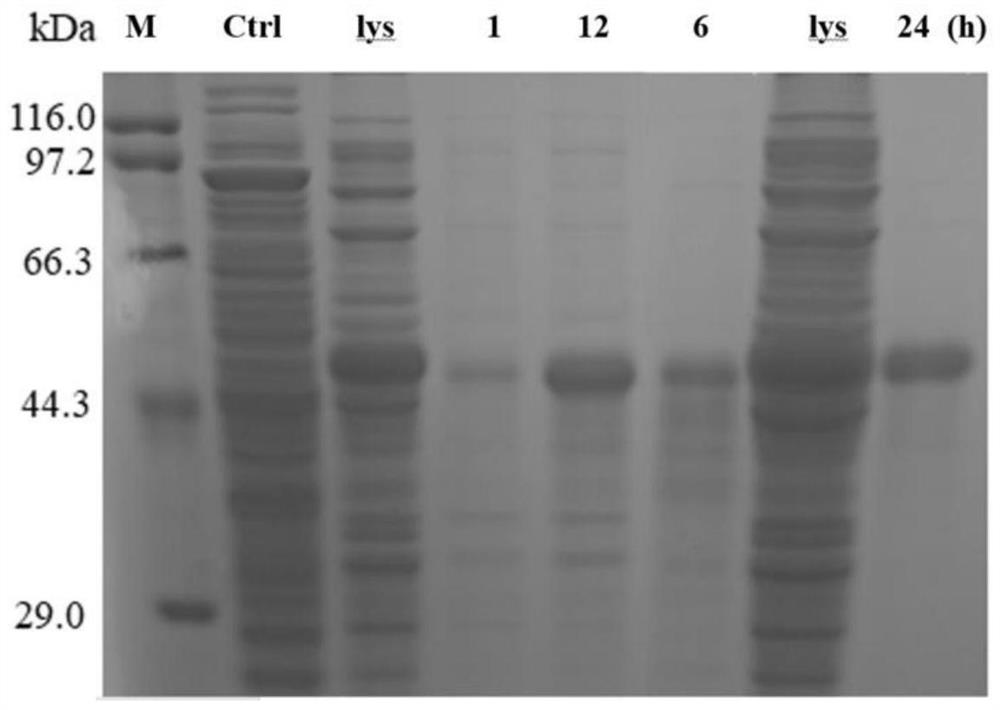
[0060] In this embodiment, the cooling coagulation method is used to purify hGH-(EAAAK) 5 - RMP16, to verify the self-purification performance and purification efficiency of the hGH prepared in Example 2. The specific operation steps are:
[0061] Concentration of 0.4-1.0 M (NH 4 ) 2 SO 4 Add to 500 μL supernatant containing soluble protein, mix well, place on ice for 30-60 min, then centrifuge at 4 °C, 12000 rpm for 15 min, separate supernatant and precipitate, and resuspend the precipitate in Tris- HCl (50 mM, pH 8.0) buffer. Place the resuspended Tris-HCl buffer at 0°C for 1, 2, 12, and 24 hours, observe the coacervate that appears at different temperatures at different times, and absorb the coacervate, which is the target protein hGH-(EAAAK) 5 - RMP16. Transfer the coacervate to a new EP tube and save a portion of the purified sample for SDS-PAGE analysis.
[0062] figure 2 The SDS-PAGE g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com