Method for improving activity of dye decolorizing peroxidase
A technology for decolorization of peroxidase and dyes, applied in the field of enzyme engineering, can solve the problems of inconvenient operation, increased cost, etc., and achieve the effect of improving the specific enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
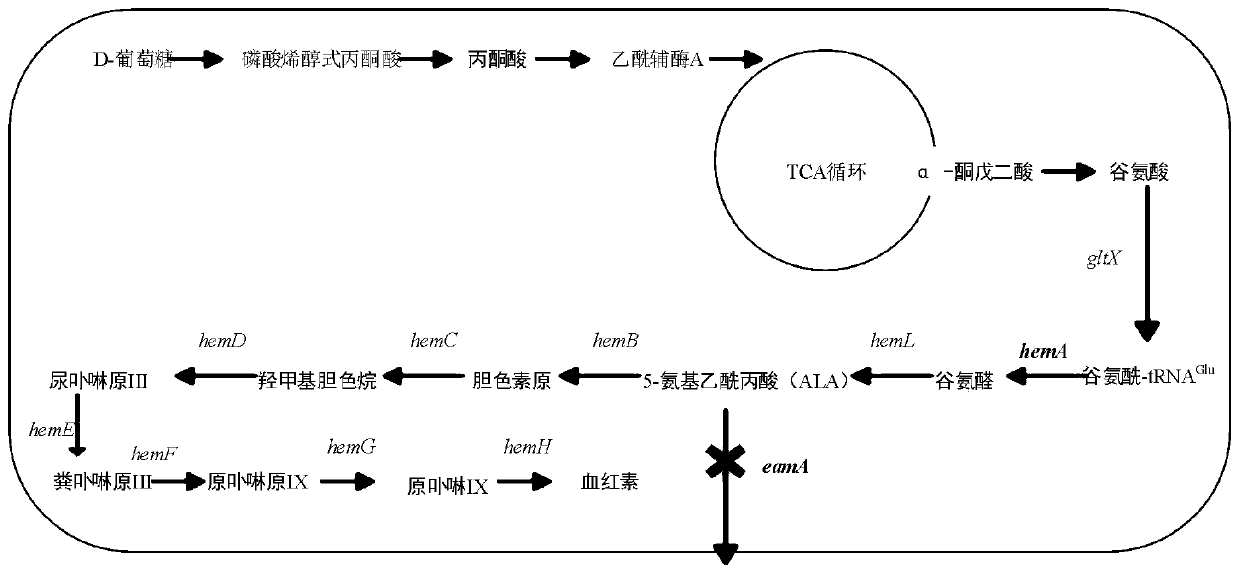
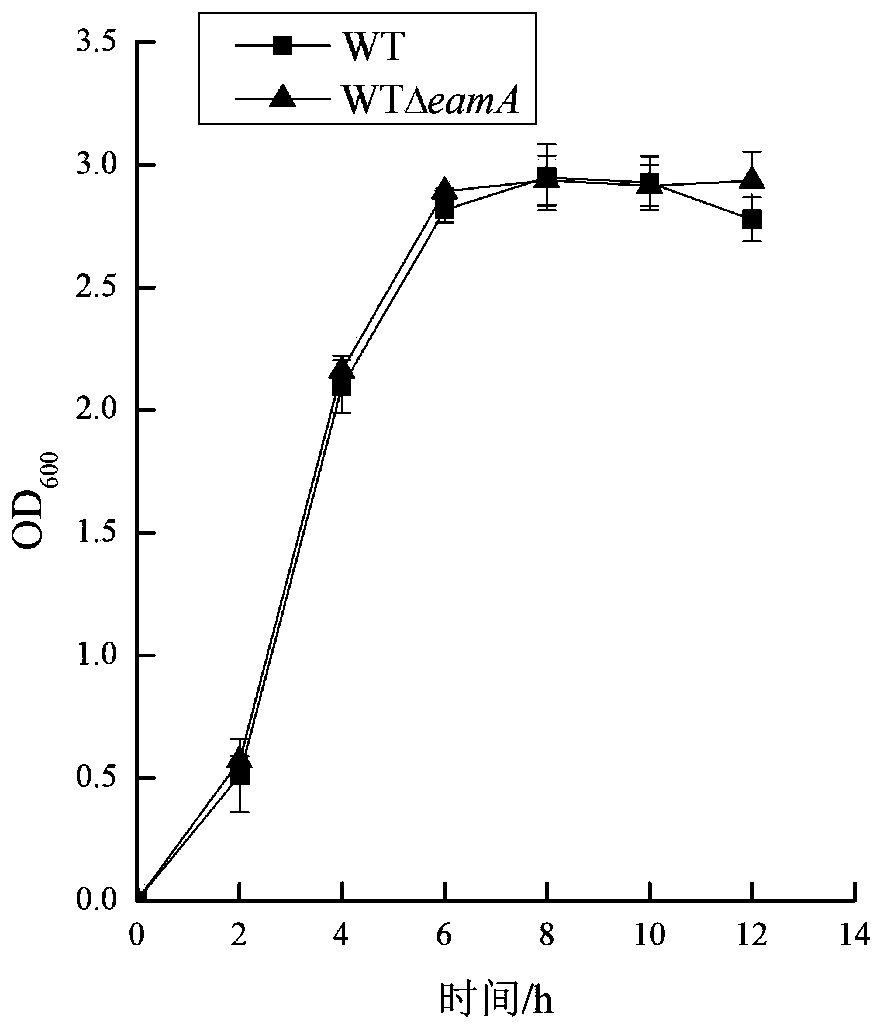
[0024] Example 1 Construction and Growth Detection of Knockout Bacteria WT△eamA
[0025] Using Escherichia coli BL21 (DE3) as the starting strain WT, the upstream and downstream homology arms of 500 bp were respectively amplified in the eamA gene (reference GenBank: AM946981.2: 1569528-1570427). Using the pKD4 plasmid as a template, the Kan resistance sequence was amplified. Linearize the pET-22b plasmid with double enzyme digestion and use Novizyme The Multis One Step Cloning Kit connects the 500bp homology arms of the upstream and downstream and the three fragments of the Kan resistance sequence to the vector pET-22b, and the ligated products are transferred to the competent state of E.coliJM109, and screened on the LB plate added with Amp and Kan The positive bacteria obtained the plasmid pEUKD. Using pEUKD as a template, PCR amplification was performed to obtain a targeting fragment containing upstream and downstream homology arms of the eamA gene and Kan resistance.
...
Embodiment 2
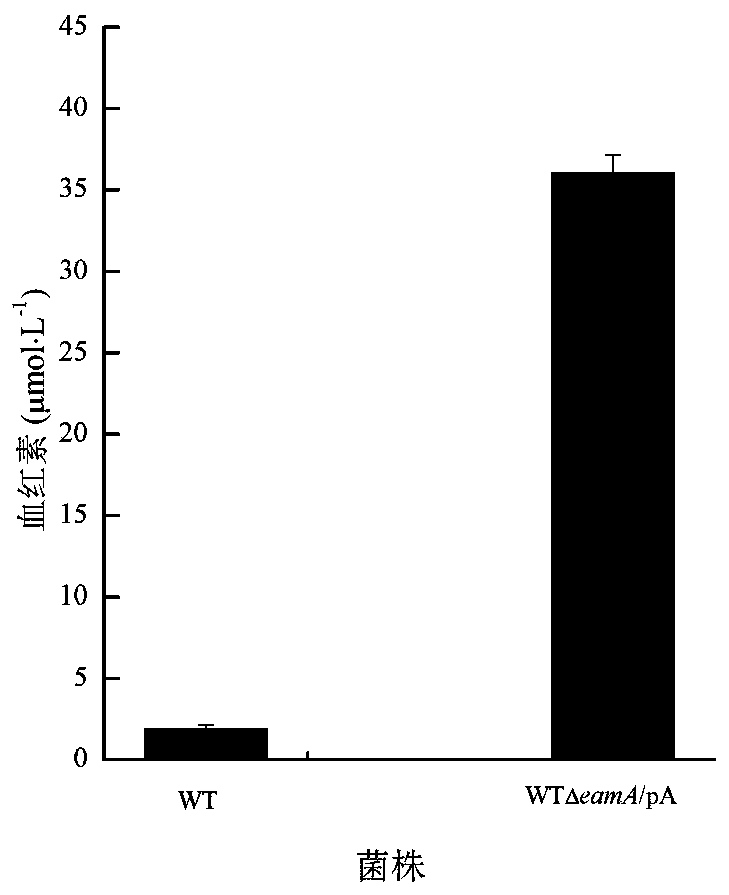
[0027] Example 2 Construction of Engineering Bacteria WT△eamA / pA and Determination of Heme Content
[0028] The glutamyl-tRNA reductase hemA gene (GenBank: AM946981.2: 1251856-1253112) was connected to the pET28a vector and introduced into the knockout bacteria WT△eamA. The specific import method was to transfer the connected product to Into the competent cell WT△eamA: Add the ligation product to the competent cell melted after ice bath, mix well, and let it stand on ice for 30 minutes; heat shock in 42°C water bath for 90 seconds, quickly put it on ice for 5 minutes, and then add 800 μL of LB liquid medium; 37°C shaker 200r min -1 , recovered for 45 minutes, centrifuged, and the centrifuged bacterial solution was spread on the LB plate added with Kan, and the engineered bacteria WT△eamA / pA was screened, and the hemoglobin concentration of WT and WT△eamA / pA was detected by fluorescence method, specifically: : Cultivate WT and WT△eamA / pA on LB medium at 37°C and 200r / min for 8...
Embodiment 3
[0029] Example 3 Construction, expression and recombinase enzyme activity determination of Escherichia coli genetically engineered bacteria WT△eamA / pAD
[0030] The hemA gene encoding glutamyl-tRNA reductase (GenBank: AM946981.2:1251856-1253112) was combined with the gene encoding dye decolorization peroxidase DyP (GenBank: AAZ57111.1) to obtain the recombinant vector pAD. Introduce the recombinant vector pAD into WT and WT△eamA respectively to obtain Escherichia coli genetically engineered bacteria WT / pAD and WT△eamA / pAD, and activate and culture the above-mentioned Escherichia coli genetically engineered bacteria at 30-37°C and 200r / min for 8-14h Afterwards, according to the 1-4% inoculum size, transfer to culture in the LB liquid medium containing 50 μ g / mL Kan antibiotic, when the OD of the bacterium 600 =0.6-0.8, add IPTG at a final concentration of 0.1-0.5mmol / L, and induce at 30-37°C for 8h. Suspend the cells with phosphate buffer (20mmol / L, pH=7.4), sonicate the cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com