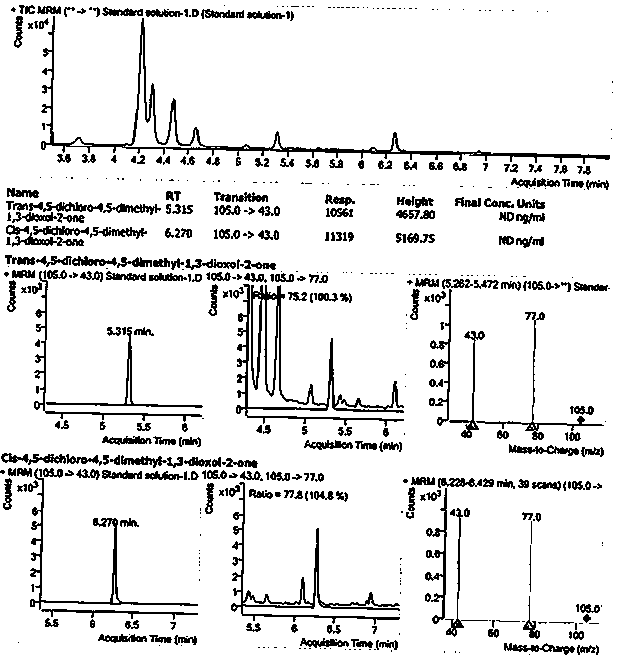
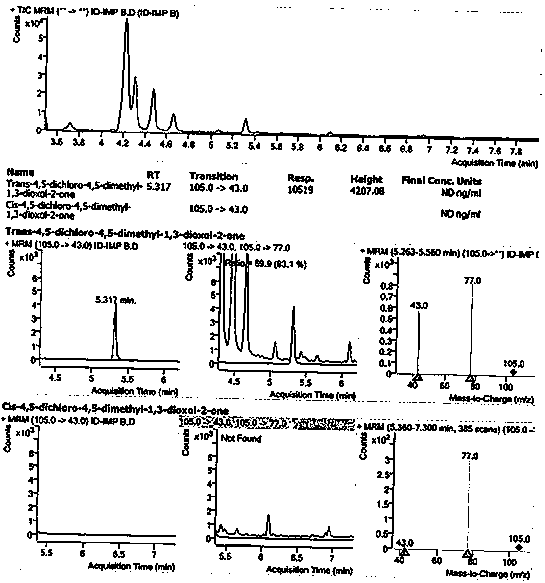
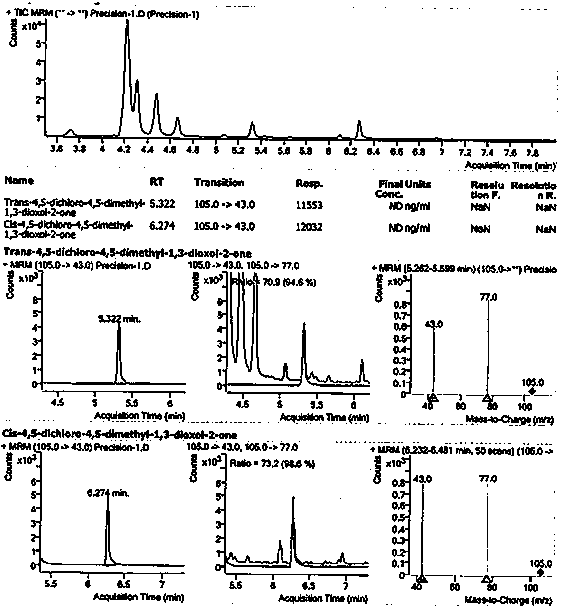
Method for detecting 1, 3-dioxolane impurities
A dioxolane and detection method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effect of low quantification limit and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 detection method
[0061] Instrument conditions and reagents
[0062] Instruments: gas chromatograph, autosampler, Agilent GC\MS QQQ 7000D equipped with EI ion source, electronic analytical balance
[0063] Chromatographic column: capillary column with (5%-phenyl)-methyl polysiloxane stationary liquid (such as: Agilent HP-5MS, 30 m x 0.25 mm, 0.25 μm, or a column with equivalent performance)
[0064] Column temperature: the initial temperature is 80°C, keep it for 3 minutes, raise the temperature to 200°C at a rate of 15°C per minute, and keep it for 5 minutes;
[0065] Inlet temperature: 150°C Carrier gas: He
[0066] Split mode: Split carrier gas flow rate: 1mL / min
[0067] Split ratio: 10:1 GC run time: 16 min
[0068] Injection volume: 2µL
[0069] Mass Spectrometry Conditions:
[0070]
[0071] Reagents and reference substances
[0072] Dichloromethane: AR and above
[0073] Trans-4,5-dichloro-4,5-dimethyl-1,3-dioxolan-2-one (impurity B): pu...
Embodiment 2
[0094] Embodiment 2 System Applicability
[0095] The system suitability is realized by the S / N of impurity B and impurity C in the sensitivity solution and the RSD of the peak areas of impurity B and impurity C in the standard solution, and the S / N of impurity B and impurity C in the sensitivity solution is required All should not be less than 10; 6. The RSD of the peak areas of impurity B and impurity C in the standard solution should not be greater than 10.0%; in order to confirm the system suitability during the sequence operation, every 8 hours or sequence Finally, enter 1 reference solution, and the RSD of the peak areas of impurity B and impurity C in the reference solution for 6 consecutive reference solutions after the requirement should not exceed 10.0%; if it exceeds this range, an evaluation investigation should be carried out.
[0096] Solution preparation
[0097] Diluent: dichloromethane;
[0098] Blank solution: diluent;
[0099] According to the preparation...
Embodiment 3
[0103] Example 3 specificity
[0104] Specificity is achieved by determining whether the blank solution interferes with the detection of impurity B and impurity C, and the separation between impurity B and impurity C and adjacent peaks in the selective solution; the blank solution is required to have no interference to the detection, and the selective solution The separation between impurity B, impurity C and adjacent impurity peaks should not be less than 1.5.
[0105] Solution preparation
[0106] Diluent: dichloromethane;
[0107] Blank solution: diluent;
[0108] Impurity B stock solution: refer to Example 2 impurity B stock solution;
[0109] Impurity C stock solution: refer to Example 2 Impurity C stock solution;
[0110] Reference substance mixed stock solution: refer to the reference substance mixed stock solution under the item of Example 2;
[0111] Impurity B positioning solution: Accurately measure 1.0mL of impurity B stock solution, place it in a 100ml volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com