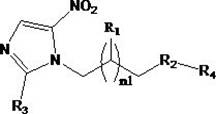
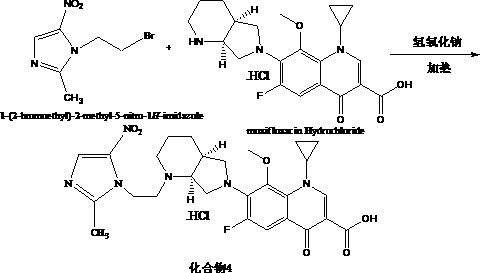
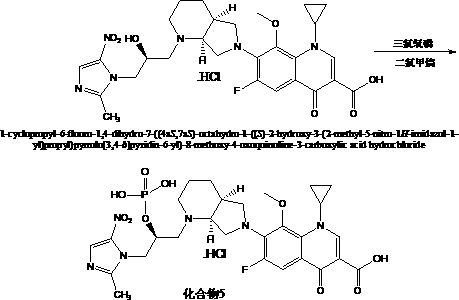
Anti-infective drug and preparation method and application thereof
An anti-infection and drug technology, applied in the field of medicine, can solve the problems of drug toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Preparation of 2-methyl-5-nitro-1-(((S)-oxiran-2-yl)methyl)1H-imidazole
[0080]
[0081] Weigh 100g of left-ornidazole and add it into 500ml of dichloromethane, stir and dissolve, cool down to -10~-15℃; weigh 36.5g of sodium hydroxide, add 500ml of water, stir and dissolve, let cool, slowly add dropwise into dichloromethane, Control the temperature not to exceed -10°C. After the addition, keep it warm at 0-5°C for 2 hours, separate the dichloromethane layer, extract the water layer with 100ml of dichloromethane, combine the dichloromethane layers, and add 100ml of water to the dichloromethane layer for washing. Twice, add an appropriate amount of anhydrous sodium sulfate to dry, filter, and concentrate the filtrate under reduced pressure to obtain 75.7 g of a brown oil.
Embodiment 2
[0082] Example 2: Preparation of 2-methyl-5-nitro-1-(((R)-oxiran-2-yl)methyl)1H-imidazole
[0083]
[0084] Weigh 100g of dex-ornidazole and add it into 500ml of dichloromethane, stir and dissolve, cool down to -10~-15°C; weigh 36.5g of sodium hydroxide, add 500ml of water, stir and dissolve, let it cool, and slowly add it dropwise into dichloromethane, Control the temperature not to exceed -10°C. After the addition, keep it warm at 0-5°C for 2 hours, separate the dichloromethane layer, extract the water layer with 100ml of dichloromethane, combine the dichloromethane layers, and add 100ml of water to the dichloromethane layer for washing. Twice, add an appropriate amount of anhydrous sodium sulfate to dry, filter, and concentrate the filtrate under reduced pressure to obtain 72.5 g of a brown oil.
Embodiment 3
[0085] Example 3: Preparation of 2-methyl-5-nitro-1-((oxiran-2-yl)methyl)1H-imidazole
[0086]
[0087] Weigh 100g of ornidazole and add it into 500ml of dichloromethane, stir and dissolve, cool down to -10~-15℃; weigh 36.5g of sodium hydroxide, add 500ml of water, stir and dissolve, let cool, slowly add dropwise into dichloromethane, control The temperature should not exceed -10°C. After the addition, keep it warm at 0-5°C for 2 hours, separate the dichloromethane layer, extract the water layer with 100ml of dichloromethane, combine the dichloromethane layers, and add 100ml of water to the dichloromethane layer to wash for 2 hours. Once, add an appropriate amount of anhydrous sodium sulfate to dry, filter, and concentrate the filtrate under reduced pressure to obtain 76.3 g of a brown oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com