Device and method for synthesizing cyclopentanol from cyclopentene
A technology of cyclopentene and cyclopentanol, which is applied in the field of cyclopentene synthesis cyclopentanol equipment, can solve the problems of low conversion rate of cyclopentene, difficulty in industrialization, high equipment requirements, etc., and achieve easy operation, simple process, and easy operation low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A method for synthesizing cyclopentanol from cyclopentene, comprising the following steps:
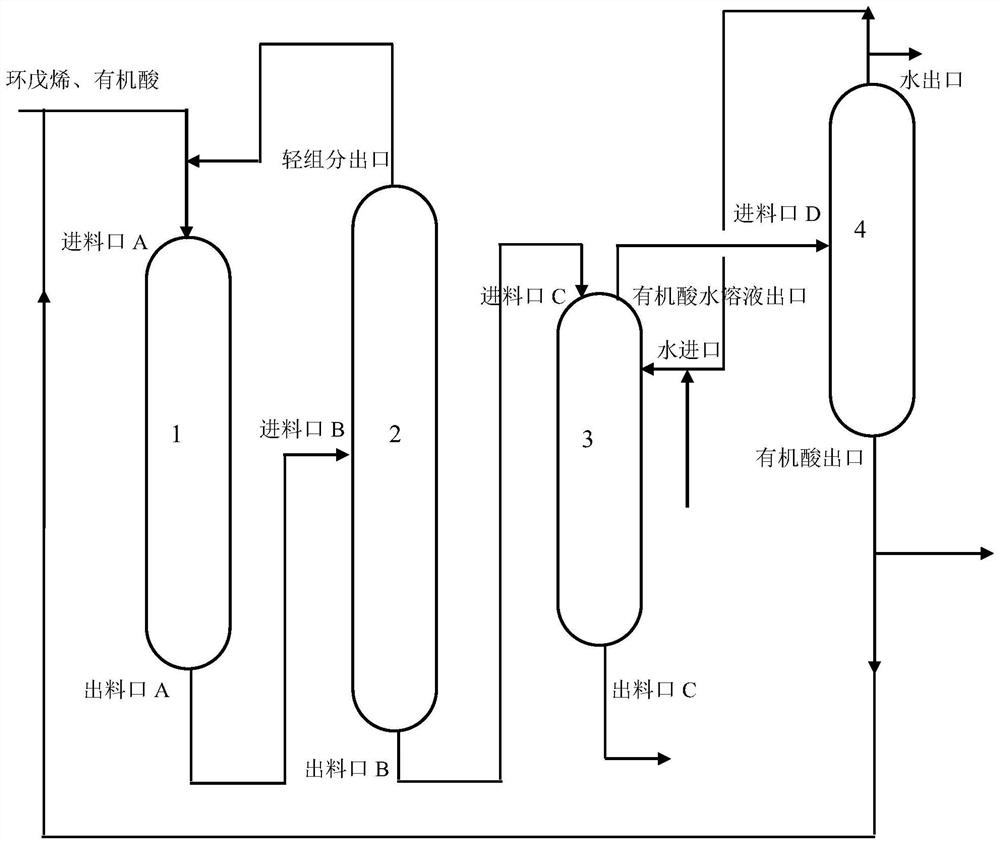
[0054] (1) Pre-reaction: cyclopentene and acetic acid (the molar ratio of acetic acid and cyclopentene is 1.2:1) is fed from feed port A (space velocity is 5h -1 ) enters the reactor, reacts under the catalysis of the KC119 type acidic resin catalyst in the reactor to obtain cyclopentyl acetate, and the reaction product is cyclopentyl acetate generated by the reaction, unreacted ring KC119 type acidic resin catalyst pentyl Alkenes and unreacted acetic acid, the reaction product is discharged from the outlet A and enters the catalytic distillation tower through the inlet B; the reaction temperature of the reactor is 40°C, and the reaction pressure is 0.1MPa.
[0055] (2) catalytic distillation reaction: the reaction product obtained in step (1) (space velocity is 0.5h -1 ) into the catalytic distillation tower, unreacted cyclopentene, unreacted acetic acid continue to react in t...
Embodiment 2
[0060] A method for synthesizing cyclopentanol from cyclopentene, comprising the following steps:
[0061] (1) Pre-reaction: cyclopentene and acetic acid (the molar ratio of acetic acid and cyclopentene is 5:1) is fed from feed port A (space velocity is 0.5h -1 ) enters the reactor, reacts under the catalysis of the KC104 type mesoporous acidic resin catalyst in the reactor to obtain cyclopentyl acetate, and the reaction product is cyclopentyl acetate generated by the reaction, unreacted cyclopentene, Unreacted acetic acid, the reaction product is discharged from outlet A and enters the catalytic distillation tower through inlet B; the reaction temperature of the reactor is 50°C, and the reaction pressure is 0.5MPa.
[0062] (2) catalytic distillation reaction: the reaction product obtained in step (1) (space velocity is 5h -1 ) into the catalytic distillation tower, unreacted cyclopentene, unreacted acetic acid continue to react in the catalysis of the KC104 type mesoporous ...
Embodiment 3
[0067] A method for synthesizing cyclopentanol from cyclopentene, comprising the following steps:
[0068] (1) Pre-reaction: cyclopentene and propionic acid (the molar ratio of propionic acid and cyclopentene is 8:1) is fed from feed port A (space velocity is 3h -1 ) into the reactor, react under the catalysis of the KIP213 type acid resin catalyst in the reactor to obtain cyclopentyl propionate, and the reaction product is cyclopentyl propionate generated by the reaction, unreacted cyclopentene, unreacted The reacted propionic acid, the reaction product is discharged from the outlet A and enters the catalytic distillation tower through the inlet B; the reaction temperature of the reactor is 120° C., and the reaction pressure is 1 MPa.
[0069] (2) catalytic distillation reaction: the reaction product obtained in step (1) (space velocity is 5h -1 ) into the catalytic distillation tower, unreacted cyclopentene, unreacted propionic acid continue to react in the catalysis of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com