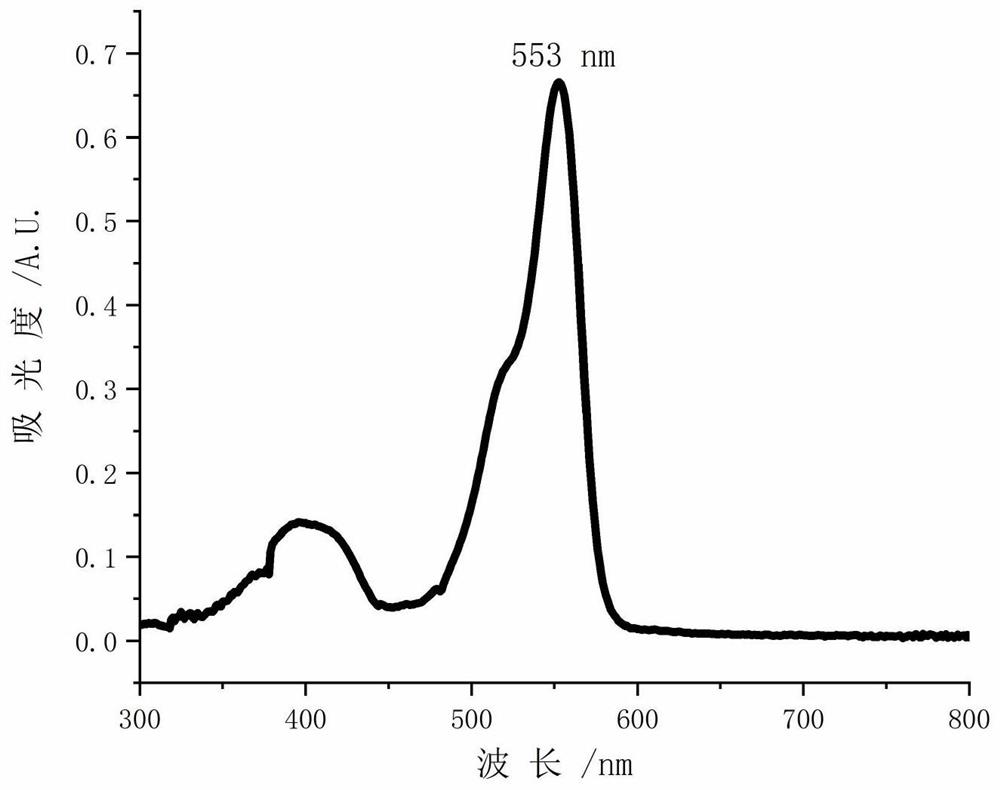
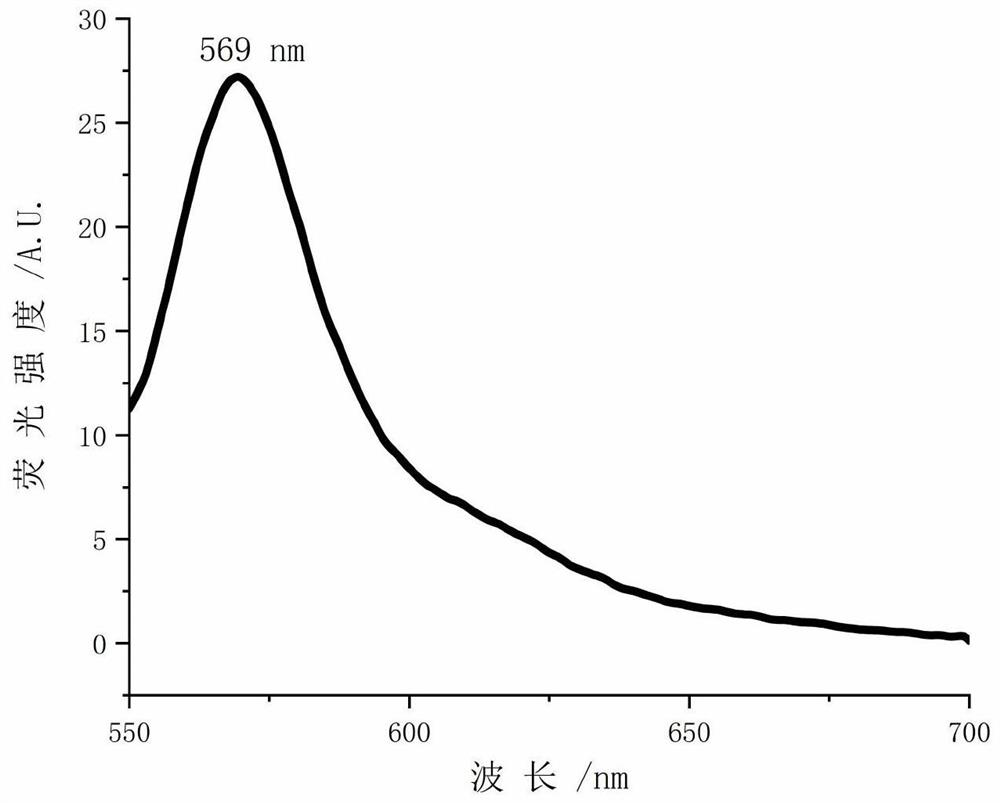
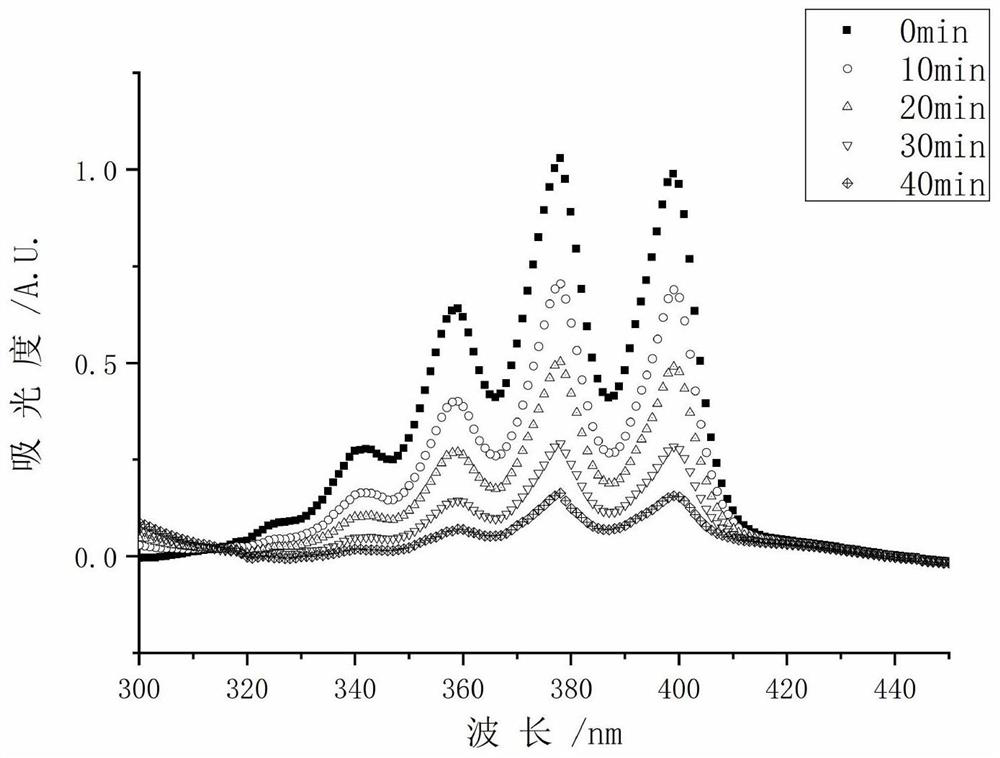
A kind of photosensitizer probe tfdb and its preparation method and application
A photosensitizer and probe technology, applied in the field of biochemistry, can solve the problems of poor photostability and low quantum yield of singlet oxygen, and achieve the effect of simple and safe preparation method, high-efficiency singlet oxygen generation ability, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Dissolve pentafluorobenzonitrile (0.3861g, 2mmol) in acetonitrile (10mL), stir at room temperature for 8min to obtain the first reaction solution, add ammonia (0.6mL) to the first reaction solution under ice-bath conditions , stirred at room temperature for 4 h, and after removing the solvent in the reaction solution, the obtained crude product was purified by column chromatography to obtain white intermediate 1;
[0033] (2) Under a nitrogen atmosphere, Dissolve Intermediate 1 (0.3509g, 1.85mmol) in ultra-dry dichloromethane (10mL) to obtain a second reaction solution, diisobutylaluminum hydride (2.2mL) in - Add dropwise to the second reaction solution at 78°C, and stir the mixture at -78°C for 4h to obtain the reaction product;
[0034] The reaction product was poured into water (30 mL) for layering, the aqueous phase was extracted with dichloromethane (10 mL×3), the combined organic phase was dried over anhydrous sodium sulfate, and concentrated to remove the org...
Embodiment 2
[0042] (1) Dissolve pentafluorobenzonitrile (0.3861g, 2mmol) in acetonitrile (9mL), stir at room temperature for 5min to obtain the first reaction solution, add ammonia (0.5mL) to the first reaction solution under ice-bath conditions , stirred at room temperature for 3.5h, and after removing the solvent in the reaction solution, the obtained crude product was purified by column chromatography to obtain white intermediate 1;
[0043](2) Under a nitrogen atmosphere, Dissolve Intermediate 1 (0.3509g, 1.85mmol) in ultra-dry dichloromethane (9mL) to obtain a second reaction solution, diisobutylaluminum hydride (2.1mL) in - Add dropwise to the second reaction solution at 75°C, and stir the mixture at -75°C for 4h to obtain the reaction product;
[0044] The reaction product was poured into water (30 mL) for layering, the aqueous phase was extracted with dichloromethane (10 mL×3), the combined organic phase was dried over anhydrous sodium sulfate, and concentrated to remove the organ...
Embodiment 3
[0054] (1) Dissolve pentafluorobenzonitrile (0.3861g, 2mmol) in acetonitrile (11mL), stir at room temperature for 10min to obtain the first reaction solution, add ammonia (0.7mL) to the first reaction solution under ice-bath conditions , stirred at room temperature for 5 h, and after removing the solvent in the reaction solution, the obtained crude product was purified by column chromatography to obtain white intermediate 1;
[0055] (2) Under a nitrogen atmosphere, Dissolve Intermediate 1 (0.3509g, 1.85mmol) in ultra-dry dichloromethane (11mL) to obtain a second reaction solution, diisobutylaluminum hydride (2.3mL) in - Add dropwise to the second reaction solution at 80°C, and stir the mixture at -80°C for 4h to obtain the reaction product;
[0056] The reaction product was poured into water (30 mL) for layering, the aqueous phase was extracted with dichloromethane (10 mL×3), the combined organic phase was dried over anhydrous sodium sulfate, and concentrated to remove the or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com