Fluorescence-oriented superoxide enhanced photosensitizer dye based on benzophenothiazine dimer as well as preparation method and application thereof
A benzophenothiazine dimer and oriented technology, which is applied in the field of biochemistry, can solve the problems of conflicts between light dose and scavenging ability, and achieve the goal of enhancing superoxide generation ability, reducing light dose, and effective scavenging effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation method of the fluorescence-guided superoxide-enhanced photosensitizer based on the benzophenothiazine dimer of this embodiment comprises the following steps:
[0052] (1) Synthetic intermediate 1: N-propargyl-1-naphthylamine
[0053] The synthetic route is as follows:
[0054]
[0055] Dissolve 1-naphthylamine (20mmol) and propyne bromide (20mmol) in 20mL of DMF, add potassium carbonate (20mmol) to it with stirring, and heat the reaction at 90°C for 4h with stirring until the reaction is complete, stop the reaction, and use After the solvent was distilled off under reduced pressure, it was redissolved with dichloromethane (50 mL), 100 mL of water was added thereto, and extracted with dichloromethane (200 mL x 3) to obtain an organic layer solution. The organic solvent was dried and removed under reduced pressure to obtain a crude product, which was purified by silica gel chromatography, and the eluent was petroleum ether: dichloromethane=6:1 (v / v) to...
Embodiment 2 2
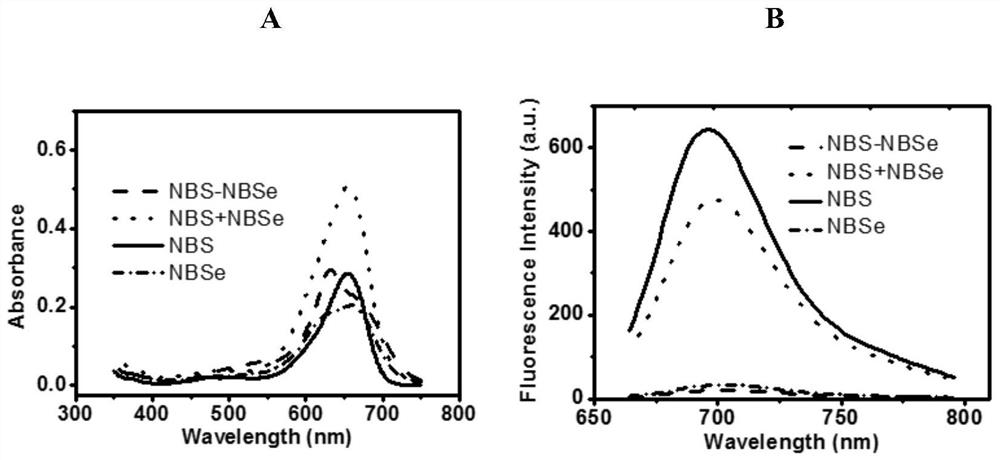
[0070] Example 2 Comparison of absorption and fluorescence quenching effects of dimers and monomers:
[0071] Prepare dimer structure example 1 and monomeric intermediates 2 and 3 starting mother solutions at 3 mM, and test their absorption and fluorescence spectra at a concentration of 10 μM in acetonitrile. Such as figure 2 As shown in A, the dimer structure of NBS-NBSe exhibits only double the absorbance intensity compared to the monomer, whereas if no dimer quenching occurs, the two-molecule structure can produce nearly double the absorbance, as in the direct Superimposition of NBS+NBSe in solution is shown. Similarly, the fluorescence spectrum shows ( figure 2 B), compared with the highly fluorescent Nile Nile Blue and Nile Selenide structures, the dimer structure NBS-NBSe exhibited a significant fluorescence quenching effect, while the stacked NBS and NBSe in pure solution only Exhibited slightly reduced fluorescence. This experiment confirmed that the dimer strate...
Embodiment 3
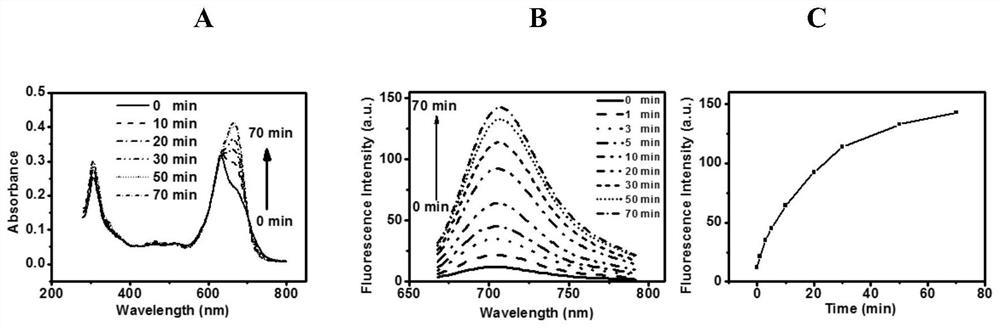
[0072] Embodiment 3 The absorption and fluorescence regulation effect of dimer:
[0073] The dimer structure in this example is connected by disulfide bonds, aiming to realize the function of fluorescent indicator that can be regulated by glutathione overexpressed in the tumor. Such as image 3 As shown in A, under the action of 50 equivalents of glutathione, the dimer structure exhibits a process of enhanced absorption over time (double peaks gradually become single peaks). At the same time, along with the disaggregation process of the dimer, the dimer structure NBS-NBSe simultaneously exhibited an obvious fluorescence recovery process (such as image 3 As shown in B), it can recover to about 10 times in 30 minutes (such as image 3 C shown). It is proved that the structure can effectively realize the fluorescence regulation under glutathione, and achieve the function as a tumor fluorescence recognizer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com