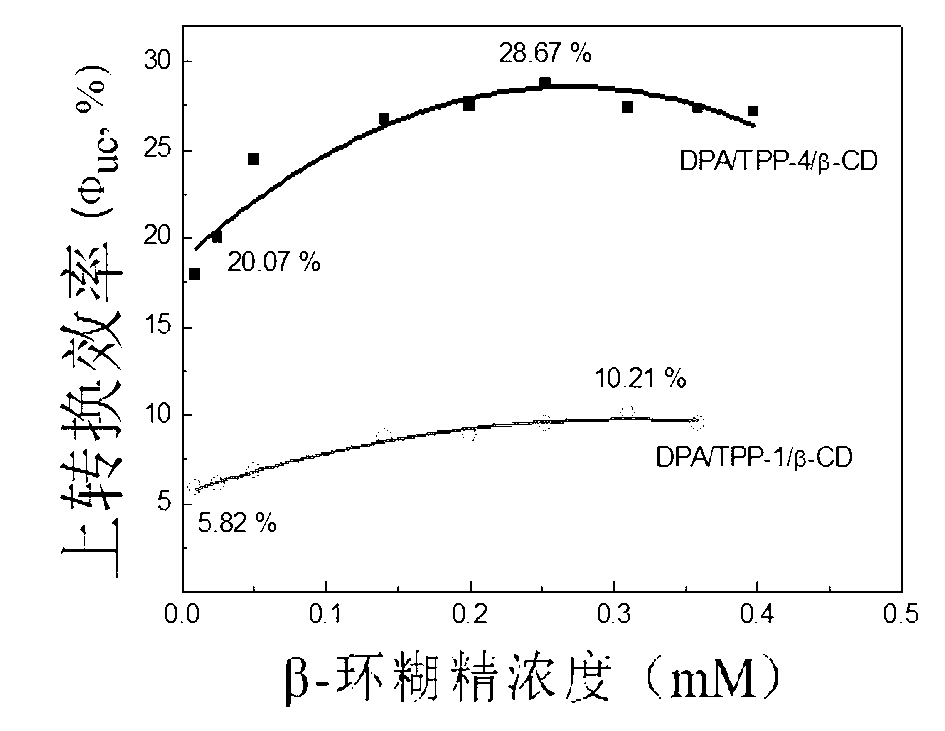
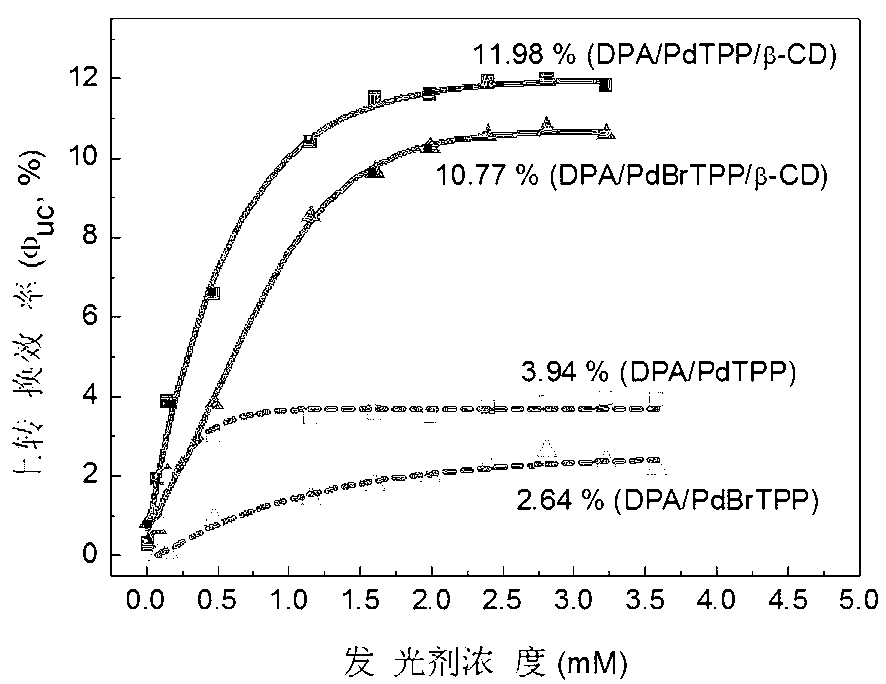
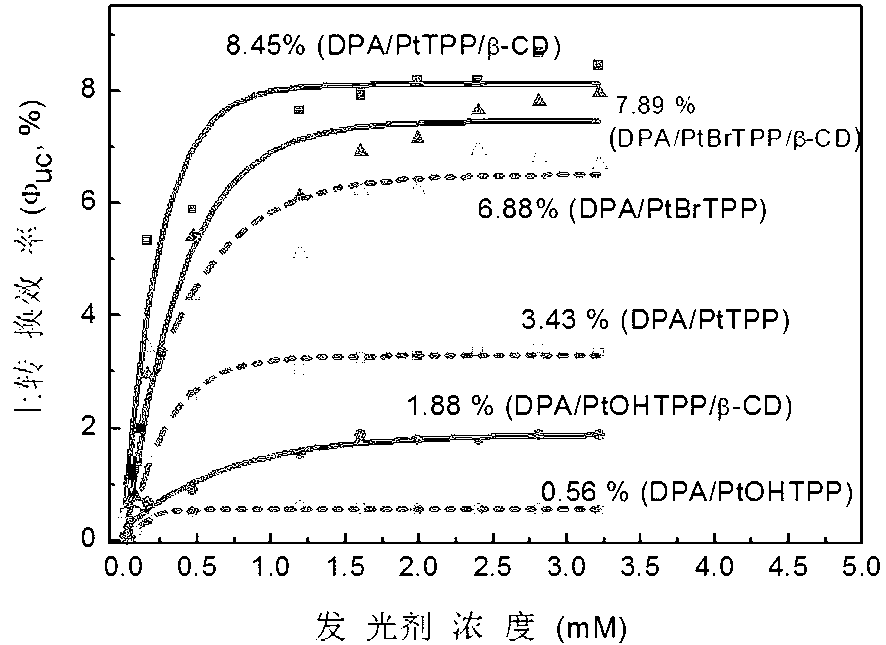
Weak light frequency up-conversion ternary supramolecular composite system
A supramolecular and system technology, applied in the directions of luminescent materials, chemical instruments and methods, can solve the problems of low up-conversion efficiency of material systems, and achieve the effect of improving up-conversion quantum yield, increasing the probability of vertical transition, and inhibiting aggregation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of β-cyclodextrin / 2-chloro-9,10-di(4-methylphenyl)anthracene (β-cyclodextrin / CDTA)
[0031] (1) 2-Chloro-9,10-bis(4-methylphenyl)-9,10-dihydroxyanthracene (compound 1 ) synthesis: burn a 250ml three-necked round-bottom flask to replace N 2 gas three times at N 2 Under air protection, inject 4-bromotoluene (5.5 mL, 0.041 mol) in anhydrous tetrahydrofuran solution (40 mL), cool the system to -78 °C, slowly add t-BuLi (25 mL, 0.062 mol, 1.6M), colorless A white precipitate gradually appeared in the transparent solution. After reacting at -78°C for 30 minutes, the reaction was continued at room temperature for 30 minutes. The system was then cooled to -78°C, and 2-chloroanthraquinone (4 g, 0.016 mol) was injected into THF (60 mL). , the white turbid liquid turned into a wine red turbid liquid. After the dropwise addition, the system was reacted at -78°C for 30 minutes, and the reaction was naturally warmed to room temperature and stirred for 24 hours...
Embodiment 2
[0036] Example 2 Preparation of β-cyclodextrin / 2-cyano-9,10-di(4-tolyl)anthracene (abbreviation: β-cyclodextrin / DTACN)
[0037] (1) Synthesis of 2-cyano-9,10-di(4-tolyl)anthracene (DTACN): under nitrogen atmosphere, add CDTA (0.72 g, 0.0018 mol) prepared in Example 1, CuCN (0.97 g, 0.0108 mol) and NMP (30 mL), stirred, heated to reflux, reacted for 96 hours, the yellow-green turbid liquid gradually became clear, and then a black solid precipitated out. Cool down to 70°C and add FeCl 3 (5.8 g) in concentrated HCl (10 mL) was stirred for 3 hours, filtered with suction, washed with water and dichloromethane, and spin-dried to obtain a black solid, which was purified by silica gel column chromatography (dichloromethane:petroleum ether=0~1:6) , to obtain 0.46 g of a yellow-green solid, which is the luminescent molecule DTACN, with a yield of 66.7%. 1 H NMR (CDCl 3 , ppm): δ=2.55 (s, 6H, CH 3 ), 7.26-7.46d, 12H, Ar-H), 7.76-7.80 (d, 3H, Ar-H), 8.16 (s, 1H, Ar-H ). MS (m / z): 383;...
Embodiment 3
[0040] Example 3 Preparation of β-cyclodextrin / 2-chloro-9,10-di(β-naphthyl)anthracene (abbreviation: β-cyclodextrin / CDNA)
[0041] (1) 2-Chloro-9,10-di(β-naphthyl)-9,10-dihydroxyanthracene (compound 2 ) Synthetic: method is as described in the step (1) in the embodiment one, only need change 4-bromotoluene into 2-bromonaphthalene, obtain tan solid; Column chromatographic purification (dichloromethane: sherwood oil=1:4 ) to obtain a beige solid compound 2 , the molecular structure formula is:
[0042]
[0043] (2) Synthesis of 2-chloro-9,10-di(β-naphthyl)anthracene (CDNA): The method is as described in step (2) in Example 1, and the compound 1 switch to compound 2 , to obtain 1.08 g light yellow needle-like solid cDNA, yield: 24.83%. 1 H NMR (CDCl 3 , ppm): δ = 7.42-8.11 (m, 21H, Ar-H); MS(m / z): 464; Elemental analysis (%): Caled. C, 87.82%; H, 4.55%; Cl, 7.62% ; Found C, 87.86%; H, 4.65%; Cl, 7.49%. The molecular structural formula is:
[0044]
[0045] (3) P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com