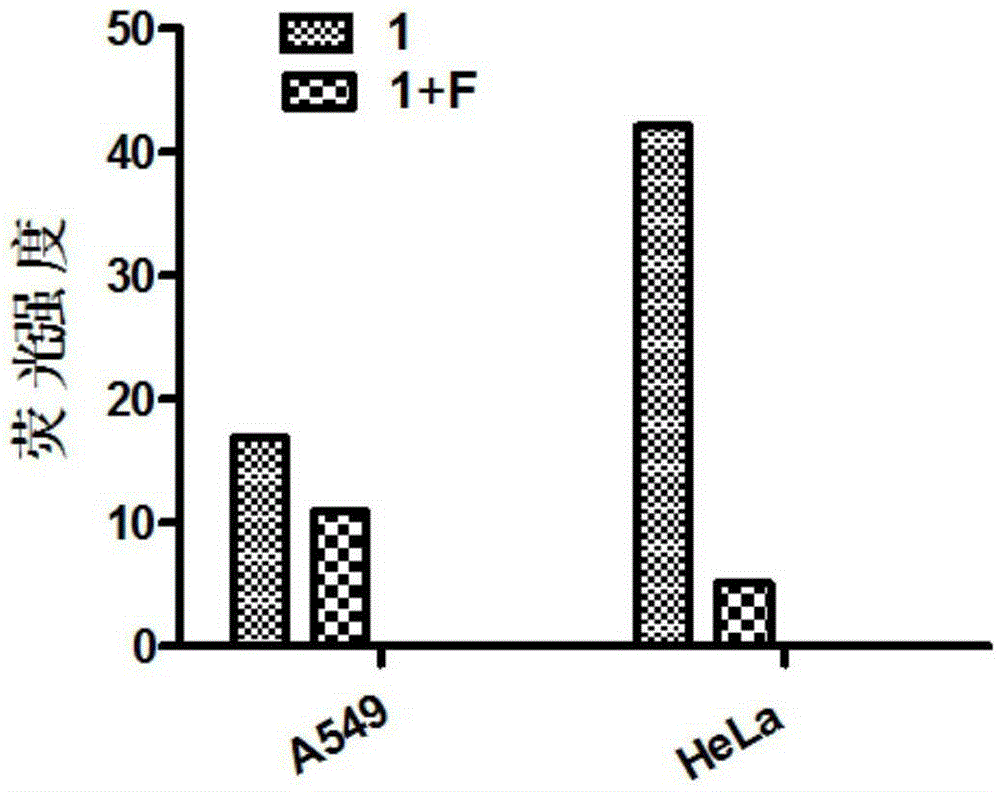
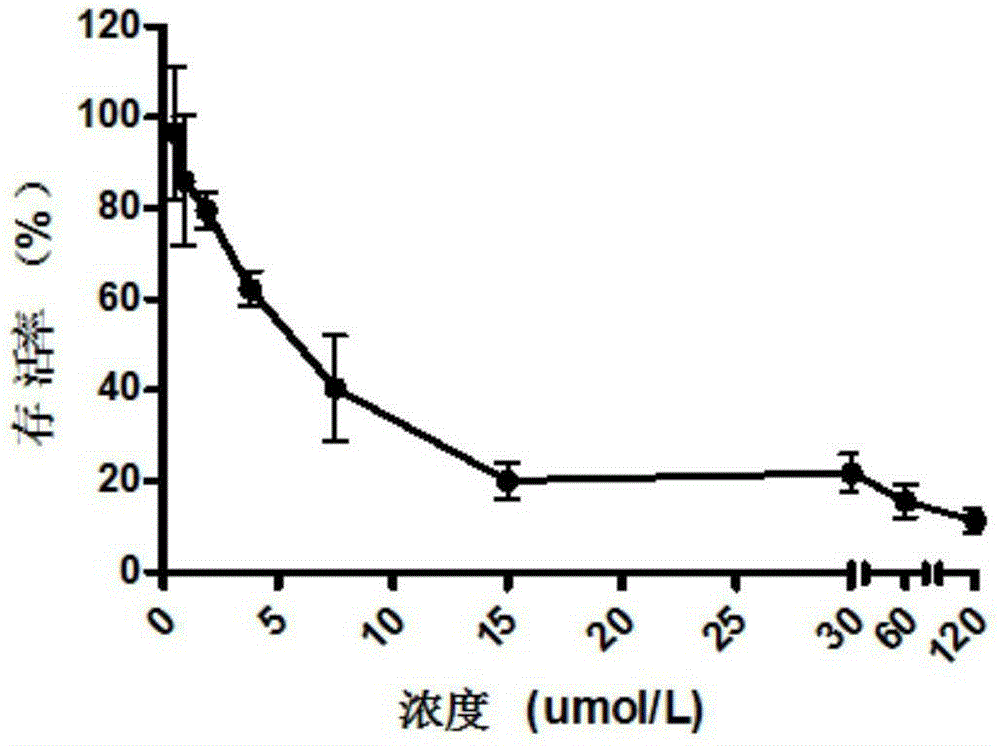
Reduction-sensitive-type water-soluble molecularly-targeted photosensitizer and preparation method and application thereof
A molecularly targeted, water-soluble technology, applied in the fields of biology and medicine, can solve the problem of poor photodynamic tumor inhibitory activity, achieve the effects of ensuring photodynamic activity, improving bioavailability, and reducing phagocytosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
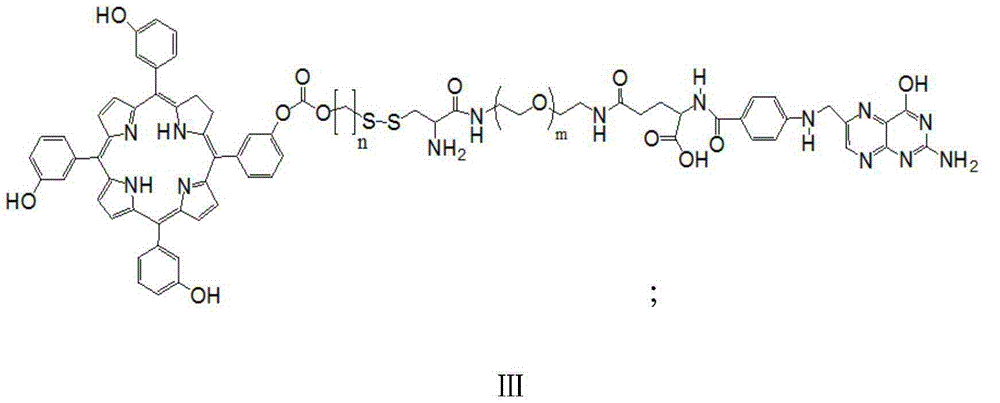
[0054] The reduction-sensitive water-soluble molecular targeting photosensitizer described in the present invention is composed of a porphine structural unit with a hydroxyl group on the middle benzene ring through a carbonate bond, a disulfide bond, and a PEG amine and a folic acid structural unit combined by an amide bond. of conjugates.
[0055] The preparation method of the reduction-sensitive water-soluble molecular targeting photosensitizer of the present invention is carried out according to the following steps:
[0056] (1) First mTHPC, 2-(n-hydroxyalkyl) dithiopyridine and equimolar triphosgene condensation reaction in the dark, control the temperature and time of the reaction, purify, and obtain the porphine intermediate containing carbonate and disulfide bond Body; The general structural formula of porphine intermediate is shown in formula I:
[0057]
[0058] Among them, n=1-5.
[0059] (2) polyethylene glycol diamine (NH 2 PEGNH 2 ) and folic acid are conde...
Embodiment 2
[0070] The synthesis of embodiment 22-(2-hydroxyethyl)-dithiopyridine
[0071] Under the protection of argon, 250ml of sulfuryl chloride was added to 250ml of dry dichloromethane containing 25g of 2-mercaptopyridine, reacted at room temperature for 4h, and evaporated to dryness under reduced pressure. Newly add 200ml of dichloromethane to dissolve, add 50ml of dichloromethane (containing 17ml of 2-mercaptoethanol) dropwise to the above solution, react at 0°C for 30min, leave at room temperature overnight, evaporate to dryness under reduced pressure, add 2 times of 4-dimethylaminopyridine , stir. Column chromatography (SiO 2, dichloromethane: acetone = 40:1), to obtain 23.1g of product, yield: 54.87%. 1 HNMR (500MHz, CD 3 OD)δ9.97(s, 1H), 9.39(dd, J=20.7, 7.4Hz, 2H), 8.79(s, 1H), 5.34(t, J=4.8Hz, 2H), 4.50(t, J= 4.8Hz, 2H); MS (ESI): 188.0162 (M+1). The structural formula is as follows:
[0072]
Embodiment 3
[0073] Example 3 Porphine intermediate (5-{3-[(2-pyridinedithio)ethyl]oxycarbonyloxy}phenyl-10,15,20-three-(3-hydroxyphenyl)porphine) synthesis
[0074] Under the protection of argon, dissolve 1.36g of 2-dithioethyl alcohol pyridine, 1ml of triethylamine, and 0.72g of triphosgene in 200ml of dichloromethane. After reacting at room temperature for 20min, add dropwise to 15ml of acetonitrile (containing 4.5gm-THPC, 1ml of triethylamine), reacted at room temperature for 10 h, evaporated the solvent under reduced pressure, and separated by reverse-phase silica gel column chromatography (ODS-AQ, methanol) to obtain 906.5 mg, yield: 15.36%. UV-Vis (CH 3 OH,nm):414,514,540,594,648; 1 HNMR (500MHz, DMSO-d 6 )δ9.81,8.72~8.21(m,6H,CH,Pyrrole),8.21~7.06(m,20H),4.48(s,2H,CH 2, OCH 2 ),4.17(s,4H,CH 2 ,Pyrrole),3.23(s,2H,CH 2 ,CH 2 S), 1.23 (s, 2H, NH); MS (ESI): 894.1722 (M+H); HPLC: 96.6%. Its structural formula is as follows:
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com