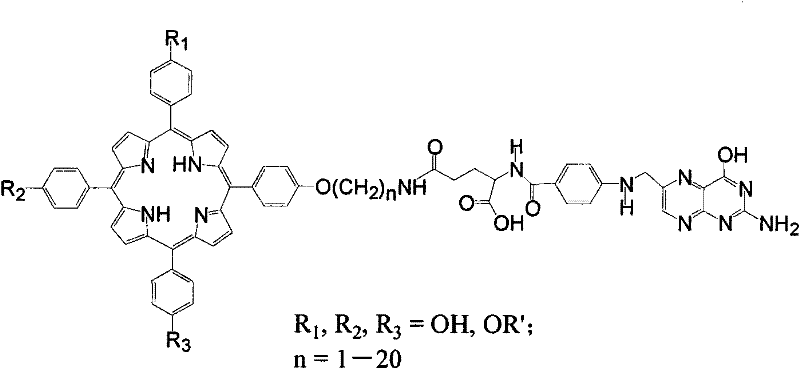
Folic acid acceptor mediated molecular targeted photosensitizer and preparation method thereof
A technology of molecular targeting and photosensitizers, applied in the field of tumor-targeting photodynamic therapy, can solve the problems of diamino compound instability, reduced bioavailability, shortened cycle time, etc., to achieve active targeting and improve stability The effect of simple and convenient preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
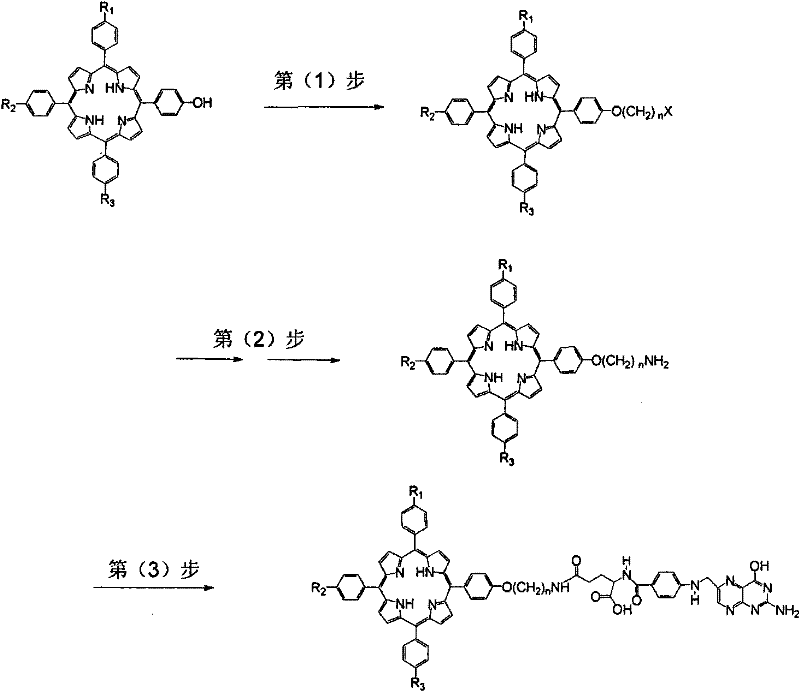
[0046] like figure 2 As shown, the preparation method of the molecularly targeted photosensitizer of the present invention comprises the following steps:
[0047] (1), monohydroxyporphyrin reacts nucleophilically with dihaloalkane under alkali catalysis to generate halogenated porphyrin; wherein, the monohydroxyporphyrin has three substituents R 1 , R 2 and R 3 The monohydroxyporphyrin, the R1, R2 and R3 are each independently a methoxy group; the dihaloalkane is C 1-20 1, n-dihaloalkane, wherein n=1-20;
[0048] (2), the halogenated porphyrin obtained by step (1) undergoes a Gabriel reaction with potassium phthalimide to generate amide porphyrin, which is then hydrolyzed with hydrazine in an aprotic solvent to generate primary amine porphyrin;
[0049] (3) The primary amino porphyrin obtained in step (2) reacts with the activated γ-carboxyl of folic acid to generate the target compound folic acid porphyrin.
experiment example 1
[0050] Experimental example 1: Synthesis of bromoporphyrin
[0051] In 110mL of N,N-dimethylformamide solution, add 16.1mmol of freshly baked potassium carbonate, 2.1mmol of monohydroxyporphyrin and 30mmol of 1,3 dibromopropane, N 2 Stir and react at 80°C for 2h, wait for the reaction liquid to cool slightly, pour it into 100mL of sodium chloride aqueous solution, let stand for 2h, filter with suction, wash with a large amount of water, wash with a small amount of 95% (volume ratio) ethanol, and dry in vacuo to obtain crude product. The crude product was separated by column chromatography (silica gel H, chloroform elution), the first purple band was collected, concentrated under reduced pressure and then recrystallized with chloroform-petroleum ether to obtain 1.77g of brominated porphyrin, yield: 88%, purple-red solid. 1 HNMR (CDCl 3 )δ: 8.85 (s, 8H, β-H), 8.77-8.21 (d, 8H, Ar-H), 7.30-7.25 (d, 8H, Ar-H), 4.34-4.13 (t, 2H, BrCH 2 ), 4.10(s, 9H, 3×OCH 3 ), 3.83-3.69 (t, 2...
experiment example 2
[0052] Experimental example 2: Synthesis of primary amine porphyrin
[0053] In 100mL of N,N-dimethylformamide solution, add 924mg of bromoporphyrin and 375mg of potassium phthalimide, stir and react at 100°C for 9h under the protection of argon, then pour the reaction solution into NaCl solution Stand in the middle for several hours, filter with suction, wash with water three times and wash twice with a small amount of ethanol, and vacuum dry to obtain a purple solid, namely the crude product of amide porphyrin. The crude product was separated by column chromatography (SiO 2 , CHCl 3 ), the second purple band was collected and concentrated to obtain 880 mg of the intermediate. After adding 50ml of tetrahydrofuran, dimethyl sulfoxide, dimethylformamide, dioxane or dichloromethane to the intermediate, add hydrazine hydrate under the protection of argon, stir and react in an oil bath at 75°C for 6 hours, pump Dry the reaction solution, then use CHCl 3 Dissolve the solid, dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com