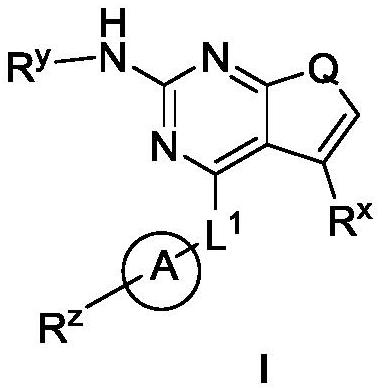
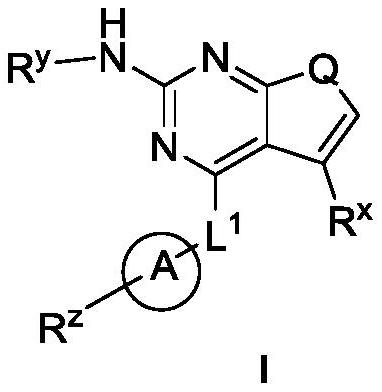
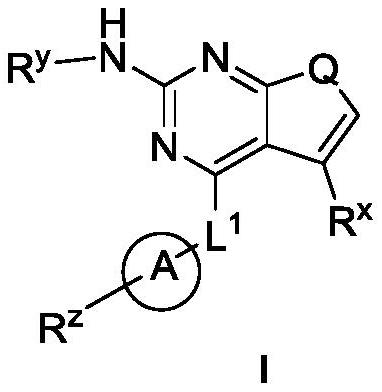
Pyrrolopyrimidine derivatives used as protein kinase inhibitors and application thereof
A protein kinase inhibitor, pyrrolopyrimidine technology, applied to pyrrolopyrimidine derivatives as protein kinase inhibitors and their application fields, can solve the problems of poor drugability, poor oral bioavailability, weak cell activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1: Preparation of 3-((2-(phenylamino)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)propane-1,2-diol (compound 1):
[0124]
[0125] 3-((2-(phenylamino)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)propane-1,2-diol of the present invention can be synthesized according to the following reaction scheme:
[0126]
[0127] Preparation of 2,4-dichloro-7-tosyl-7H-pyrrolo[2,3-d]pyrimidine (II):
[0128] 2,4-dichloro-7H-pyrrolo[2,3-d]pyrimidine (I) (2g, 10.6mmol), p-toluenesulfonyl chloride (2.2g, 11.7mmol), TEA (2.15g, 21.3 mmol) and DMAP (129mg, 1.06mmol) were added to DCM (50mL), and reacted at 25°C for 1h; TLC detected that the raw material disappeared and the reaction was complete; the solvent was spin-dried, and 200mL of water was added, and a large amount of white solid was precipitated. After washing, a white solid (II) (3.44 g, 10.1 mmol) was obtained, yield: 95%. ESI-MS m / z:342.0[M+H] + . 1 H NMR (300MHz, DMSO-d 6 )δ8.13(d, J=4.0Hz, 1H), 8.04(d, J=8.4Hz, 2H), 7.57...
Embodiment 4
[0137] Example 4: N 2 -Phenyl-N 4 Preparation of -(piperidin-4-yl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamine (compound 4):
[0138]
[0139] N of the present invention 2 -Phenyl-N 4 -(piperidin-4-yl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamine can be synthesized according to the following reaction scheme:
[0140]
[0141] Preparation of tert-butyl 4-((2-chloro-7-toluenesulfonyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate (III-a) :
[0142] 2,4-dichloro-7-tosyl-7H-pyrrolo[2,3-d]pyrimidine (II) (500mg, 1.47mmol), tert-butyl 4-aminopiperidine-1-carboxylate ( 441 mg, 2.21 mmol) and TEA (445 mg, 4.41 mmol) were added to 50 mL of i-PrOH, and reacted at 80°C for 4 h; After washing (30mL×3), the organic phase was dried over anhydrous sodium sulfate, and separated by column chromatography to obtain a white solid (III-a) (600mg, 1.19mmol), with a yield of 80.5%. ESI-MS m / z:506.3[M+H] + ;
[0143] 4-(((2-(phenylamino)-7-tosyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...
Embodiment 15
[0152] Example 15: 2-amino-1-(4-(((2-((3-fluorophenyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine Preparation of -1-yl)ethan-1-one (compound 15):
[0153]
[0154] 2-amino-1-(4-(((2-((3-fluorophenyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine- 1-base) ethane-1-one can be synthesized according to the following reaction scheme:
[0155]
[0156] N 2 -(3-fluorophenyl)-N 4 The preparation of -(piperidin-4-yl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamine (VI-b) refers to the preparation of (VI-a):
[0157] tert-Butyl(2-(4-((2-(((3-fluorophenyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl )-2-oxyethyl carbamate (VII-b) preparation:
[0158] Will N 2 -(3-fluorophenyl)-N 4 -(piperidin-4-yl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamine (VI-b) (300mg, 0.924mmol), (tert-butoxycarbonyl)glycine (240mg , 1.38mmol), HATU (702mg, 1.848mmol) and DIEA (358mg, 2.77mmol) were added to DMF (20mL), and reacted at 25°C for 4h; after the reaction was complet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com