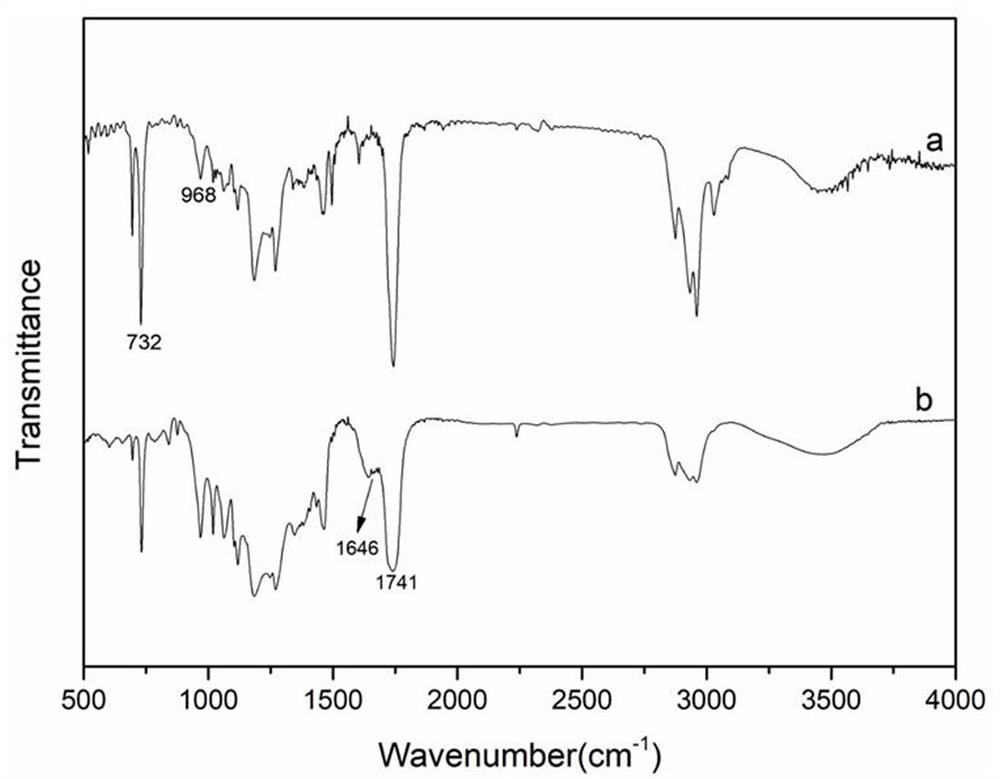
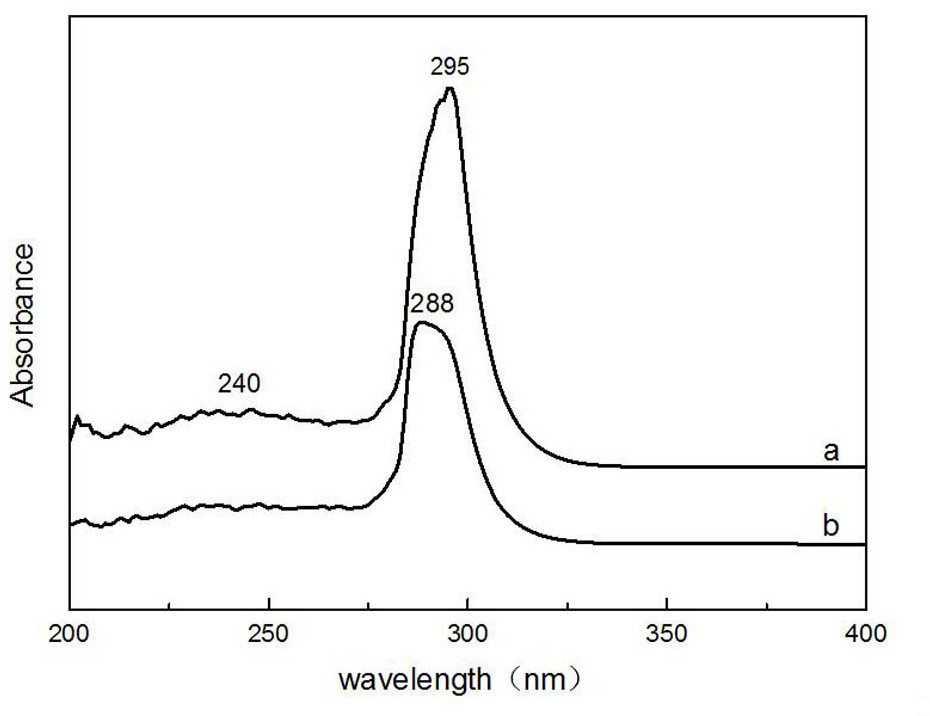
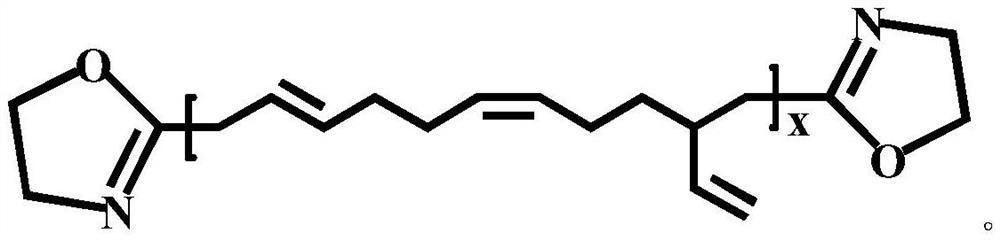
Oxazoline-terminated polybutadiene compound, and preparation method and application thereof
An oxazoline-terminated polybutadiene and polybutadiene technology, applied in recycling technology, plastic recycling and other directions, can solve problems such as environmental and human health hazards, increase in waste plastics, and reduce impact strength, and achieve environmental Friendly, mild reaction conditions, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Take by weighing 20.42g carboxyl-terminated polybutadiene and dissolve it in 50ml toluene to obtain carboxyl-terminated polybutadiene toluene solution;
[0037] (2) Slowly add 4.75 g of thionyl chloride into the above-mentioned carboxy-terminated polybutadiene toluene solution, and react with magnetic stirring at 40-50°C for 2-3 hours until the generation of bubbles stops;
[0038](3) A certain amount of ethanolamine was added into the system, and stirred for 2 hours by magnetic force, then 4A molecular sieve was added, and reacted for 3 hours at 120° C. to obtain an oxazoline-terminated polybutadiene compound. Wherein, the molar ratio of the added ethanolamine and carboxyl-terminated polybutadiene is 1:3.
Embodiment 2
[0040] (1) Take by weighing 20.42g carboxyl-terminated polybutadiene and dissolve it in 50ml toluene to obtain carboxyl-terminated polybutadiene toluene solution;
[0041] (2) Slowly add 4.75 g of thionyl chloride into the above-mentioned carboxy-terminated polybutadiene toluene solution, and react with magnetic stirring at 40-50°C for 2-3 hours until the generation of bubbles stops;
[0042] (3) A certain amount of ethanolamine was added to the system, and stirred for 2 hours by magnetic force, then 4A molecular sieve was added, and reacted for 5 hours at 120° C. to obtain an oxazoline-terminated polybutadiene compound. Wherein, the molar ratio of the added ethanolamine and carboxyl-terminated polybutadiene is 1:5.
Embodiment 3
[0044] (1) Take by weighing 20.42g carboxyl-terminated polybutadiene and dissolve it in 50ml toluene to obtain carboxyl-terminated polybutadiene toluene solution;
[0045] (2) Slowly add 4.75 g of thionyl chloride into the above-mentioned carboxy-terminated polybutadiene toluene solution, and react with magnetic stirring at 40-50°C for 2-3 hours until the generation of bubbles stops;
[0046] (3) A certain amount of ethanolamine was added into the system, and stirred for 2 hours by magnetic force, then 4A molecular sieve was added, and reacted for 7 hours at 120° C. to obtain an oxazoline-terminated polybutadiene compound. Wherein, the molar ratio of the added ethanolamine and carboxyl-terminated polybutadiene is 1:7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com