Kit for rapidly detecting bordetella pertussis and detection method
A pertussis and kit technology, which is applied in the rapid detection of pertussis bacilli and its detection field, can solve the problems of low sensitivity of serological screening, cumbersome operation, long culture period, etc., to improve thermal stability, stability, The detection steps are simple and the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Design and preparation of primers and probes
[0030] Download all genome sequences of Bordetella hallii, B. parapertussis, and B. pertussis from Genebank, and find out the difference regions of the genomes of various pathogens through multiple alignments. And the stable conserved region of the published B. pertussis genome sequence is used as the target sequence for detection, and specific detection primers and probes are designed and synthesized for the target sequence.
[0031] 2. Screening of primers and probes
[0032] Multiple sets of primers and probes designed were used to detect the genomic DNA of positive and negative controls of various pathogen samples, and after repeated experiments, the combination of primers and probes with the best sensitivity, specificity and repeatability was screened out:
[0033] Upstream primer: 5' ATGCGCGTCAACTGCGCGCCTTGTTGACGCGACGCG 3'
[0034] Downstream primer: 5' GCGATGGCCTTGTCGATGGAACCATCGACCAAG 3'
[0035] The probe seq...
Embodiment 2
[0049] 1. Evaluation of kit detection sensitivity
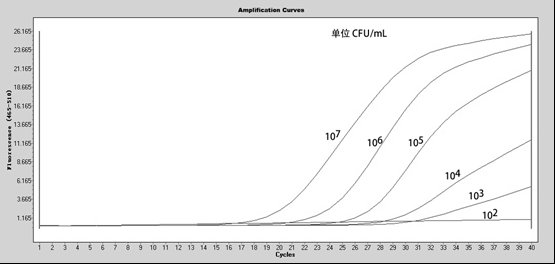
[0050] Take an appropriate amount of Bacillus pertussis bacterial liquid obtained by isolation and culture, inactivate the bacterial liquid and use turbidimetric method to measure the number of bacteria, and then dilute it successively to 10 times by 10-fold gradient dilution method. 7 、10 6 、10 5 、104 、10 3 、10 2 For bacterial suspensions on the order of CFU / mL, directly add the above samples to the amplification system for detection.
[0051] The evaluation result shows that the minimum detection limit of the kit provided by the invention to B. pertussis is 10 2 CFU / mL.
[0052] 2. Evaluation of kit detection specificity
[0053] In order to evaluate the detection specificity of the kit provided by the invention, a group of common respiratory pathogens, such as influenza A virus, influenza B virus, respiratory syncytial virus, Epstein-Barr virus, Haemophilus influenzae, were screened in the experiment from clinical s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com