Bupropion hydrochloride slow release tablets and preparation method thereof
A technology of bupropion hydrochloride and sustained-release tablets, which is applied in the direction of non-active ingredients of medical preparations, pharmaceutical formulas, oil/fat/wax non-active ingredients, etc., can solve the problems of increased incidence of epilepsy, environmental pollution, tablet Faster release and other issues, to achieve good stability of related substances, reduce clinical risk, and good release stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
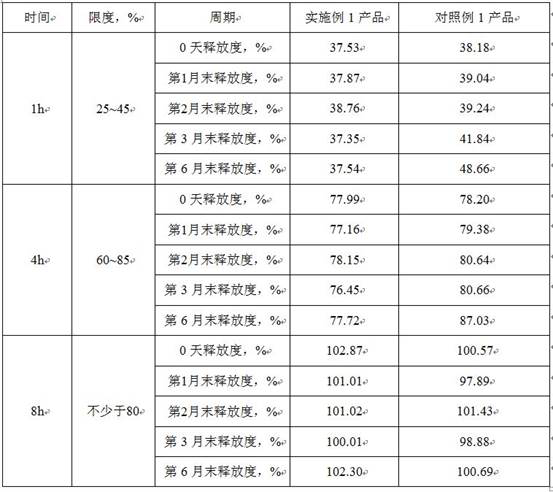
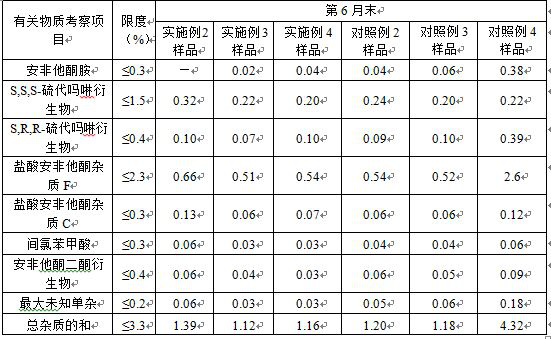
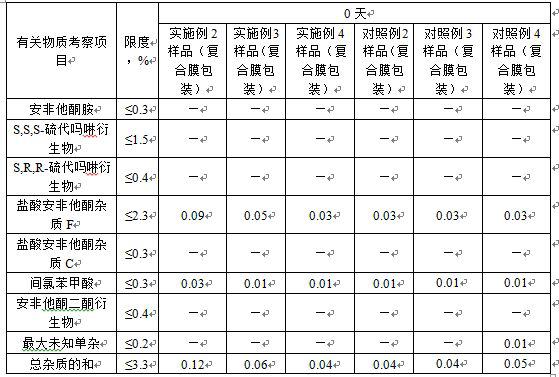
[0031] Example 1 Preparation of Bupropion Hydrochloride Sustained Release Tablets
[0032] Step 1. Preparation of Bupropion Hydrochloride Solid Dispersion
[0033] Prescription: bupropion hydrochloride 150g, carnauba wax 100g, deionized water 600g
[0034] The preparation method is as follows:
[0035] (1) Melt the prescribed amount of carnauba wax in a water bath at 90°C and set aside.
[0036] (2) Take the prescribed amount of bupropion hydrochloride plus deionized water to dissolve completely, raise the temperature to 90°C, add it to the molten waxy phase, stir evenly in a water bath at 90°C, spread the mixture on the tray, Vacuum drying at 90°C for 0.5-2 hours under reduced pressure, and the dried dispersion was passed through a 40-mesh sieve and pulverized to obtain a solid dispersion of bupropion hydrochloride.
[0037] Step 2 Preparation of Bupropion Hydrochloride Sustained Release Tablets
[0038] Prescription: 220g of bupropion hydrochloride solid dispersion obtai...
Embodiment 2
[0063] Example 2 Preparation of Bupropion Hydrochloride Sustained Release Tablets
[0064] Step 1. Preparation of Bupropion Hydrochloride Solid Dispersion
[0065] Prescription: bupropion hydrochloride 150g, carnauba wax 40g, deionized water 600g
[0066] The preparation method is as follows:
[0067] (1) Melt the carnauba wax in a water bath at 90°C and set aside.
[0068] (2) Take bupropion hydrochloride and deionized water to dissolve completely, raise the temperature to 90°C, add to the molten waxy phase, stir evenly in a water bath at 90°C, spread the mixture on a tray, and heat it at 90°C Vacuum drying under reduced pressure for 0.5 to 2 hours, and the dried dispersion was passed through a 40-mesh sieve and pulverized to obtain a solid dispersion of bupropion hydrochloride.
[0069] Step 2 Preparation of Bupropion Hydrochloride Sustained Release Tablets
[0070] Prescription: 190g of bupropion hydrochloride solid dispersion obtained in step 1, 146g of microcrystallin...
Embodiment 3
[0076] Example 3 Preparation of Bupropion Hydrochloride Sustained Release Tablets
[0077] Step 1. Preparation of Bupropion Hydrochloride Solid Dispersion
[0078] Prescription: bupropion hydrochloride 150g, carnauba wax 70g, deionized water 600g
[0079] The preparation method is as follows:
[0080] (1) Melt the carnauba wax in a water bath at 90°C and set aside.
[0081] (2) Take bupropion hydrochloride and deionized water to dissolve completely, raise the temperature to 90°C, add to the molten waxy phase, stir evenly in a water bath at 90°C, spread the mixture on a tray, and heat it at 90°C Vacuum drying under reduced pressure for 0.5 to 2 hours, and the dried dispersion was passed through a 40-mesh sieve and pulverized to obtain a solid dispersion of bupropion hydrochloride.
[0082] Step 2 Preparation of Bupropion Hydrochloride Sustained Release Tablets
[0083] Prescription: 250g of bupropion hydrochloride solid dispersion obtained in step 1, 22g of microcrystalline...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com