Bone remodeling regulating polypeptides and application
A technology for regulating and osteoporosis, which is applied in the direction of peptides containing affinity tags, peptides containing His tags, peptides, etc., to achieve the effects of simple synthesis, low cost and moderate half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
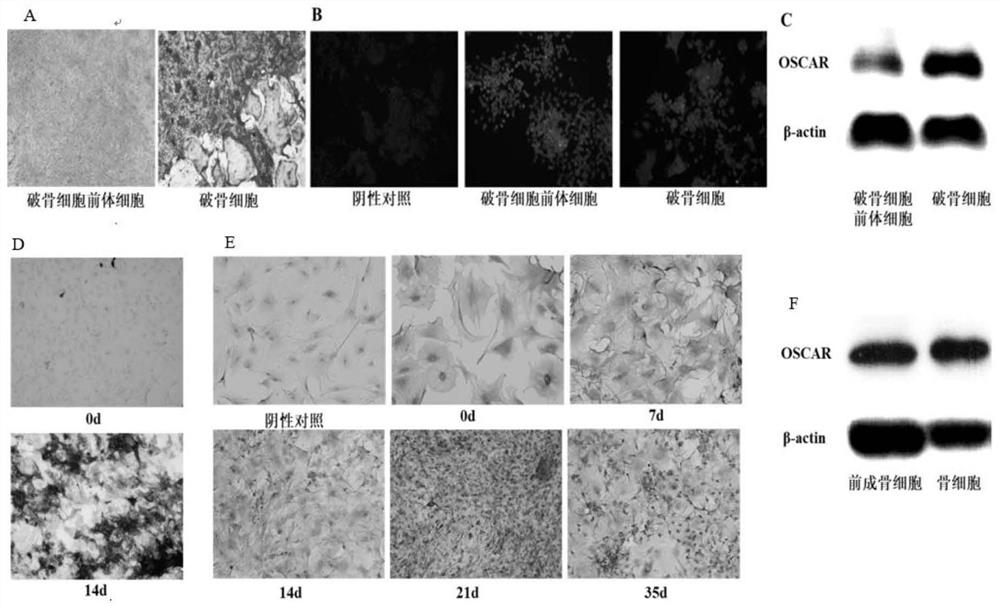
[0055] Determine the expression of collagen receptor OSCAR on osteoclasts and osteoblasts.
[0056] 1) Aseptically isolate the bilateral femur and tibia of wild-type female mice, and repeatedly wash the bone marrow cavity with the culture medium to obtain bone marrow cells. After culturing in complete α-MEM medium containing M-CSF (30ng / mL) for 24 hours, aspirate the unpasted After centrifugation, the parietal cells were resuspended, and then cultured in α-MEM complete medium containing M-CSF (30ng / mL) for 3 days to obtain adherent mouse primary bone marrow mononuclear macrophages (osteoclast precursors) cells), M-CSF (50ng / mL) and RANKL (100ng / mL) induced differentiation into osteoclasts, which were identified by TRAP staining (figure 1 A), by OSCAR immunofluorescence ( figure 1 B) and Western blot experiment ( figure 1 C) determining that osteoclast precursor cells and osteoclasts express the collagen receptor OSCAR;
[0057] 2) Aseptically isolate the skull of wild-type m...
Embodiment 2
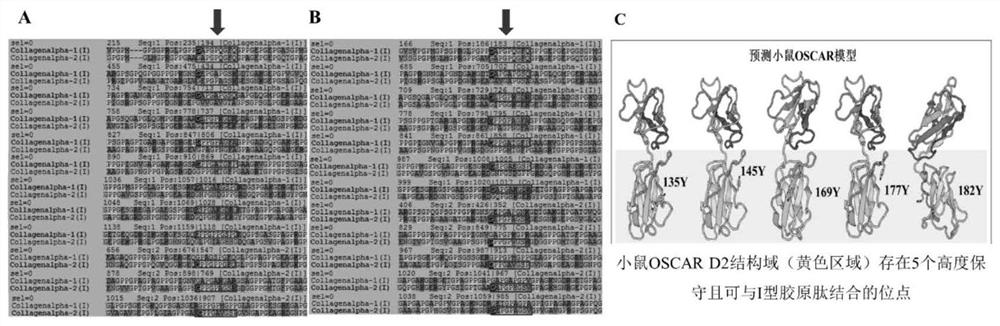
[0059] to humans ( figure 2 A) and mice ( figure 2 B) Multiple sequence alignment of type Ⅰ collagen α1 subunit and α2 subunit to find the sequence on type Ⅰ collagen that may bind to the collagen receptor OSCAR ( figure 2 A and B box parts, the upper sequence in each group of sequences represents Col1a1, and the lower sequence represents Col1a2). And by predicting the protein structure of mouse OSCAR (mOSCAR), we found that there are 5 highly conserved amino acid sites that can bind to collagen molecules ( figure 2 C).
Embodiment 3
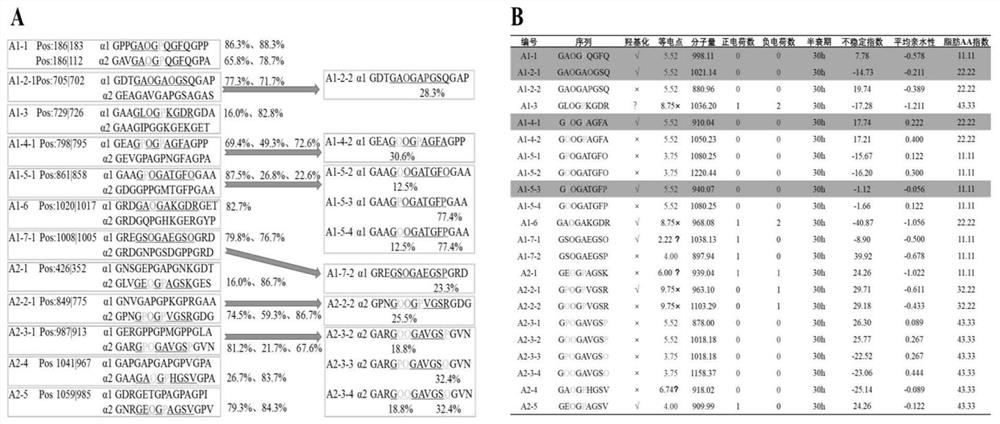
[0061] Based on the prediction of physical and chemical properties and functions (isoelectric point, molecular weight, number of positive and negative charges, half-life, instability index, average hydrophilicity, fatty amino acid index), screen 4 sequences derived from type Ⅰ collagen with stable performance and 1 sequence Sequences derived from type II and III collagens were synthesized from the screened sequences derived from type I collagen and sequences derived from type II and III collagens by solid-phase peptide synthesis (ie, five bone remodeling regulatory polypeptides were synthesized).
[0062] 1) By analyzing the hydroxylation level of amino acids in the protein sequence of mouse type Ⅰ collagen that may bind to the collagen receptor OSCAR ( image 3 A), and analyze the isoelectric point of the above sequence, the number of positive and negative charge residues, the number of atoms, the half-life, the instability coefficient, the average hydrophilicity and the aliph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com