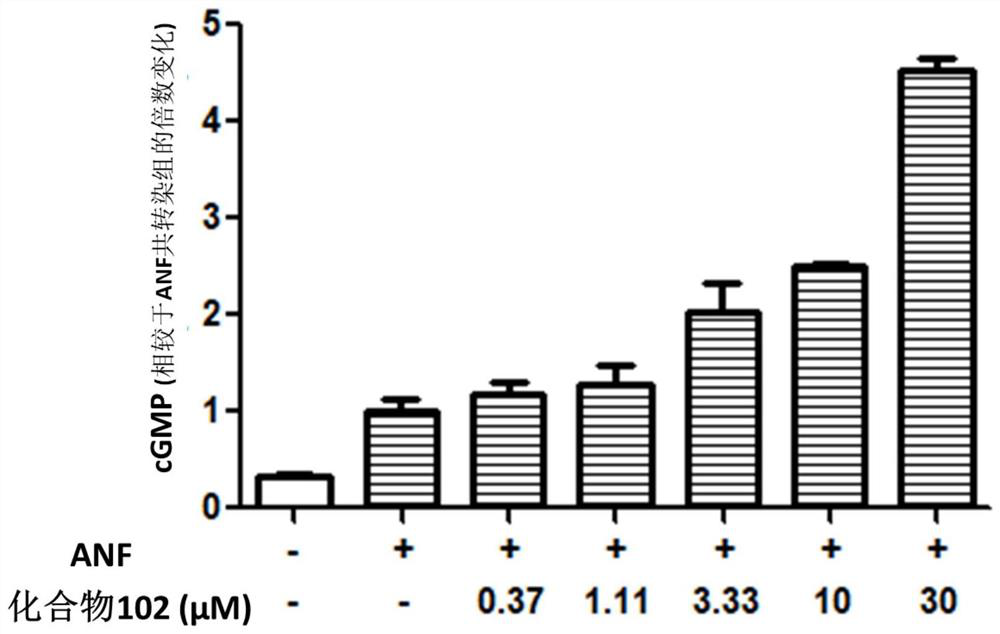
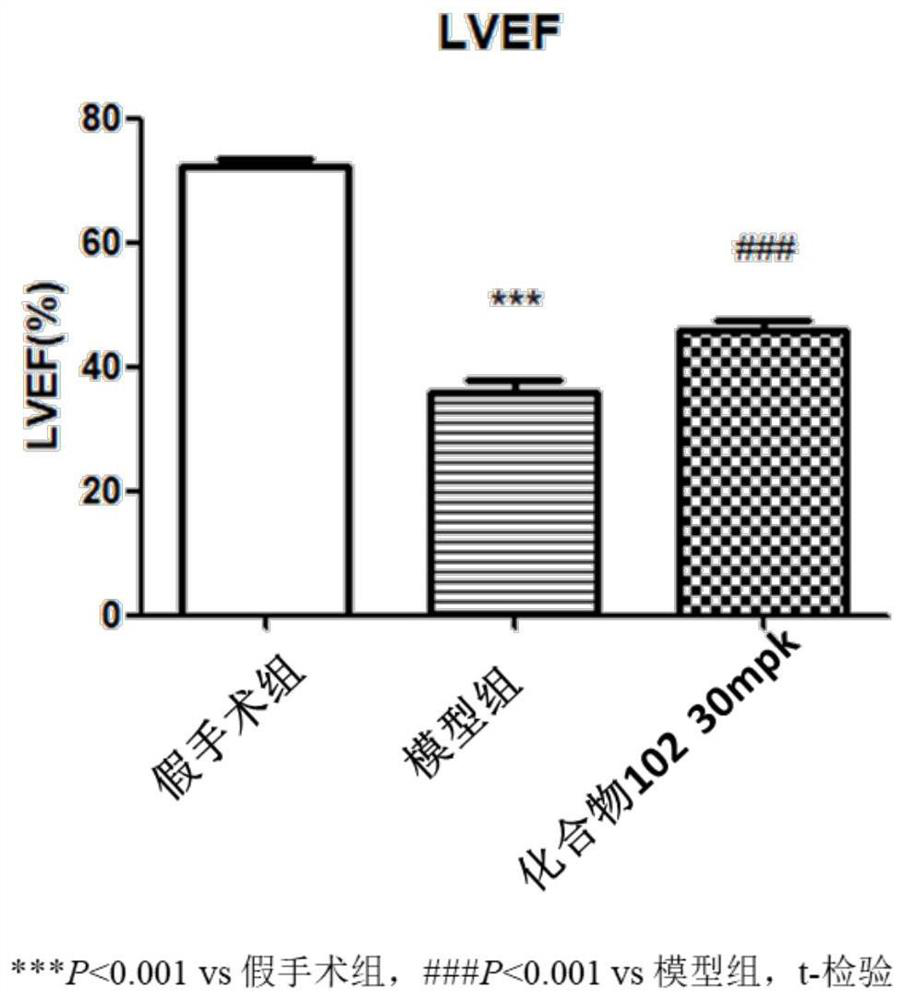
Use of phosphodiesterase inhibitor
A phosphodiesterase and inhibitor technology, applied in the field of medicine, can solve the problems of weakened myocardial protection ability and increased expression level, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0250] Preparation Example 1: Synthesis of intermediate 4,6-dichloro-2-oxo-1,2-dihydro-1,7-naphthyridine-3-carbonitrile
[0251] Step 1: Synthesis of 6-chloro-2H-pyrido[3,4-d][1,3]oxazine-2,4(1H)-dione
[0252]
[0253] Dissolve 5-amino-2-chloroisonicotinic acid (30g, 0.1738mol, 1.0eq) in N,N-dimethylformamide (300mL), and add N,N'-carbonyl di Imidazole (48g, 0.2955mol, 1.7eq), slowly warmed to room temperature overnight. LC-MS showed that the reaction was complete, cooled to room temperature, and proceeded directly to the next step without treatment.
[0254] Step 2: Synthesis of 6-chloro-4-hydroxy-2-oxo-1,2-dihydro-1,7-naphthyridine-3-carbonitrile
[0255]
[0256] Add triethylamine (35.182g, 0.3478mol, 2eq) and ethyl cyanoacetate (19.665g, 0.1738mol) to the above reaction solution, react at 150°C for 3h, LC-MS monitors that the reaction is complete, the reaction solution is cooled to room temperature, and Concentrate under reduced pressure, add water (200mL), adjus...
preparation example 2
[0260] Preparation Example 2: Intermediate 6-ethyl-4-chloro-2-oxo-1,2-dihydro-1,7-naphthalene-3-carbonitrile and 2,4-dichloro-6-ethane Synthesis of 1,7-naphthyridine-3-carbonitrile
[0261] Step 1: Synthesis of methyl 6-ethyl-3-(cyanoacetamido)-1-pyridine-4-carboxylate
[0262]
[0263] The intermediate 6-ethyl-3-amino-1-pyridine-4-carboxylic acid methyl ester (131g, 727.13mmol, 1.0eq) was dissolved in dichloromethane (1.31L), and cyanoacetic acid ( 74.22g, 872.56mmol, 1.2eq), EDCI (209.07g, 1090.70mmol, 1.5eq) was added in batches, and reacted at 25°C for 2 hours. LC-MS detected that the reaction was complete. Add H to the reaction solution 2 O (1.5L), liquid separation, organic phase with H 2 O (2×800mL), dried over anhydrous sodium sulfate, suction filtered, the filtrate was concentrated, and the crude product was slurried with methyl tert-butyl ether (500mL) to obtain the product (165g, yield: 91.78%).
[0264] Step 2: Synthesis of 6-ethyl-4-hydroxy-2-oxo-1,2-dihydr...
Embodiment 1
[0271] Example 1: 6-isopropyl-4-(4-methoxy-4-methylpiperidin-1-yl)-2-oxo-1,2-dihydro-1,7-diazepine Synthesis of Naphthalene-3-carbonitrile (Compound 91)
[0272] Step 1: Synthesis of 6-chloro-2H-pyrido[3,4-d][1,3]oxazine-2,4(1H)-dione
[0273]
[0274] Dissolve 5-amino-2-chloroisonicotinic acid (30g, 0.1738mol, 1.0eq) in N,N-dimethylformamide (300mL), and add N,N'-carbonyl di Imidazole (48g, 0.2955mol, 1.7eq), slowly warmed to room temperature overnight. LC-MS showed that the reaction was complete, cooled to room temperature, and proceeded directly to the next step without treatment.
[0275] Step 2: Synthesis of 6-chloro-4-hydroxy-2-oxo-1,2-dihydro-1,7-naphthyridine-3-carbonitrile
[0276]
[0277] Add triethylamine (35.182g, 0.3478mol, 2eq) and ethyl cyanoacetate (19.665g, 0.1738mol) to the above reaction solution, react at 150°C for 3h, LC-MS monitors that the reaction is complete, the reaction solution is cooled to room temperature, and Concentrate under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com