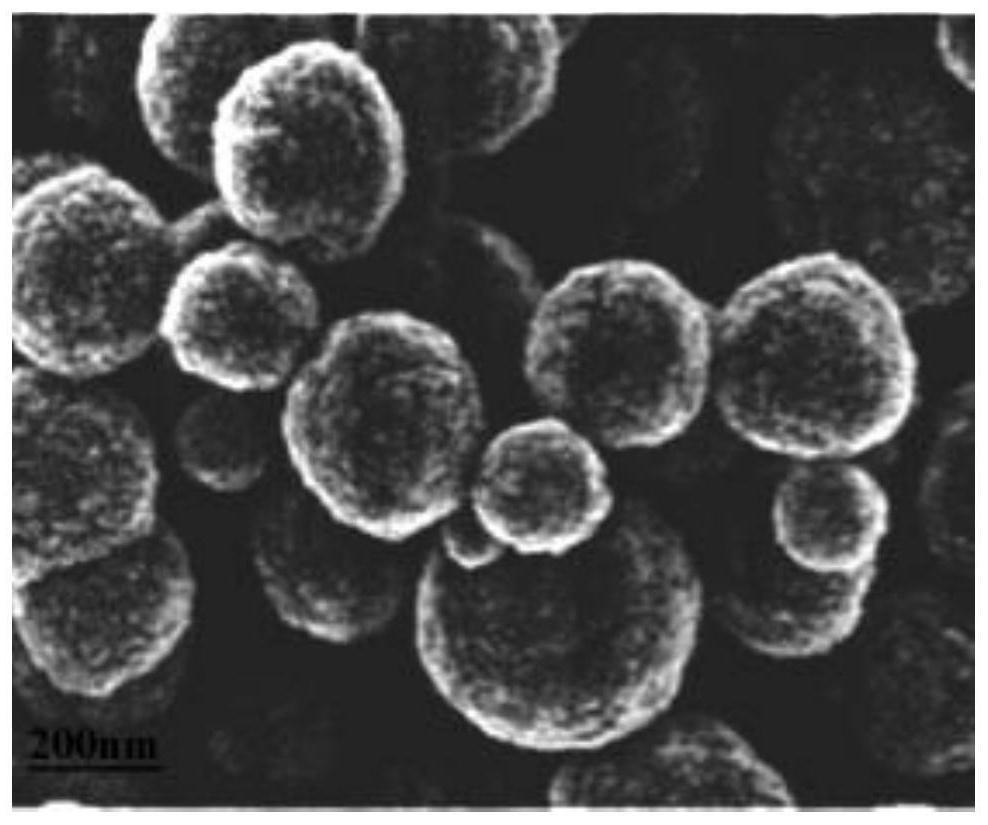
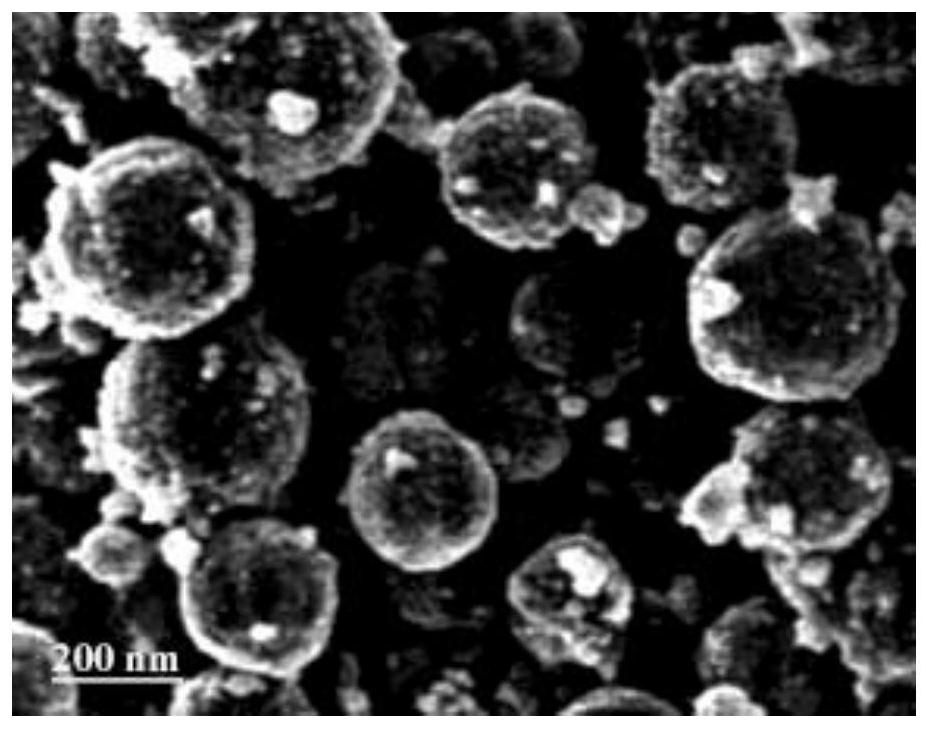
Iron-based Fischer-Tropsch synthesis catalyst and preparation method thereof
A technology for synthesis of catalysts and tropes, applied in chemical instruments and methods, preparation of liquid hydrocarbon mixtures, catalysts for physical/chemical processes, etc., can solve problems such as increasing catalyst production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] An iron-based Fischer-Tropsch synthesis catalyst, prepared by the following method:
[0026] Step 1: Disperse iron ferric oxide in deionized water, add citric acid, heat up to 80°C and stir until iron ferric oxide is completely dissolved, cool and filter out insoluble impurities, add ammonia water to the filtrate to adjust acidity and alkalinity after filtration , so that the pH is 3, then continue to stir for 3 hours after raising the temperature to 85°C to form an iron citrate complex and set aside;
[0027] Step 2: Disperse nano-polyacrylamide in deionized water, add tryptophan, heat up to 90°C, stir for 2 hours to form a modified nano-polyacrylamide gel, cool for later use;
[0028] Step 3: Add the ferric citrate complex prepared in step 1 to the gel obtained in step 2, raise the temperature to 60°C, and stir at high speed for 0.5h to obtain an iron-loaded gel, and place the obtained gel at 120°C dried in a vacuum oven;
[0029] Step 4: Place the completely dried ...
Embodiment 2
[0035] An iron-based Fischer-Tropsch synthesis catalyst, prepared by the following method:
[0036] Step 1: Disperse iron ferric oxide in deionized water, add citric acid, heat up to 80°C and stir until iron ferric oxide is completely dissolved, cool and filter out insoluble impurities, add ammonia water to the filtrate to adjust acidity and alkalinity after filtration , so that the pH was 3.5, then heated up to 85°C and continued to stir for 3 hours to form an iron citrate complex for use;
[0037] Step 2: Disperse nano-polyacrylamide in deionized water, add alanine, heat up to 90°C, stir for 2 hours to form a modified nano-polyacrylamide gel, and cool it for later use;
[0038] Step 3: Add the ferric citrate complex prepared in step 1 to the gel obtained in step 2, raise the temperature to 60°C, and stir at high speed for 0.5h to obtain an iron-loaded gel, and place the obtained gel at 120°C dried in a vacuum oven;
[0039] Step 4: Place the completely dried gel in a muffl...
Embodiment 3
[0044] An iron-based Fischer-Tropsch synthesis catalyst, prepared by the following method:
[0045] Step 1: Disperse iron oxide in deionized water, add citric acid, heat up to 80°C and stir until ferric oxide is completely dissolved, cool and filter out insoluble impurities, and add ammonia water to the filtrate to adjust acidity and alkalinity after filtration. The pH is 4, then the temperature is raised to 85°C, and the stirring is continued for 3 hours to form an iron citrate complex, which is ready for use;
[0046] Step 2: Disperse nano-polyacrylamide in deionized water, add leucine, raise the temperature to 90°C, stir for 2 hours to form a modified nano-polyacrylamide gel, cool for later use;
[0047] Step 3: Add the ferric citrate complex prepared in step 1 to the gel obtained in step 2, raise the temperature to 60°C, and stir at high speed for 0.5h to obtain an iron-loaded gel, and place the obtained gel at 120°C dried in a vacuum oven;
[0048] Step 4: Place the compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com