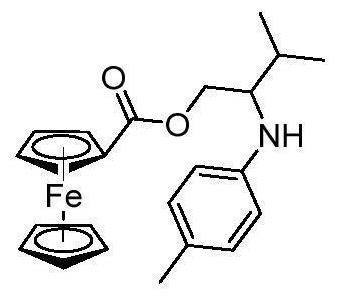
Ferrocenyl-containing arylamine compound and synthesis method thereof
A synthesis method and compound technology, applied in chemical instruments and methods, metallocenes, organic chemistry, etc., to achieve high atom economy, easy access to reagents, and simple synthesis of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
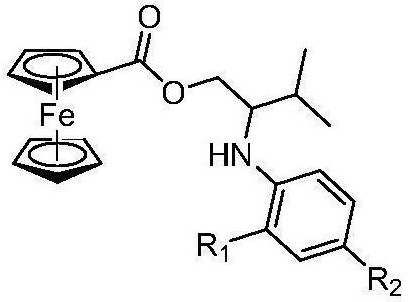
[0039] A ferrocenyl-arylamine-containing compound, the structural formula of the ferrocenyl-arylamine-containing compound is:
[0040]
[0041] The synthetic method of above-mentioned ferrocenyl arylamine compound comprises the following steps:
[0042] Prepare the ingredients first:
[0043] (s)-(4-isopropyloxazolin-2-yl)ferrocene, TCI (Shanghai) Chemical Industry Development Co., Ltd.;
[0044] 2-(trimethylsilyl)phenyl trifluoromethanesulfonate, TCI (Shanghai) Chemical Industry Development Co., Ltd.;
[0045] The specific synthesis method is:
[0046] At 60°C, 1.2mmol of (s)-(4-isopropyloxazolin-2-yl)ferrocene and 1.0mmol of trifluoromethanesulfonic acid-2-(trimethylsilyl) Phenyl ester, 2.0mmol of cesium fluoride as base and 2.0mmol of 18-crown-6-ether were reacted in 2mL of toluene solvent for 12 hours to obtain the crude product containing ferrocenyl arylamine compound; the prepared ferrocene-containing The crude product of the arylamine compound was washed with...
Embodiment 2
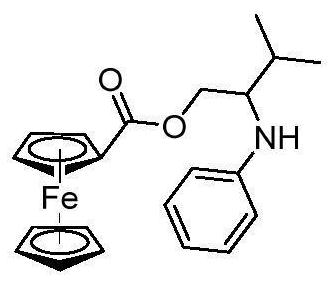
[0051] A ferrocenyl-arylamine-containing compound, the structural formula of the ferrocenyl-arylamine-containing compound is:
[0052]
[0053] The above-mentioned synthetic method containing ferrocenyl arylamine compound comprises the following steps:
[0054] Prepare ingredients:
[0055] (s)-(4-isopropyloxazolin-2-yl)ferrocene, TCI (Shanghai) Chemical Industry Development Co., Ltd.;
[0056] Trifluoromethanesulfonic acid-4-methyl-2-(trimethylsilyl)phenyl ester, TCI (Shanghai) Chemical Industry Development Co., Ltd.;
[0057] Specific synthesis method:
[0058] At 60°C, (s)-(4-isopropyloxazolin-2-yl)ferrocene 1.2mmol, trifluoromethanesulfonic acid-4-methyl-2-(trimethylsilyl ) 1.0mmol of phenyl ester, 2.0mmol of cesium fluoride as base and 2.0mmol of 18-crown-6-ether were reacted in 2mL toluene solvent for 12 hours to obtain the crude product of ferrocenyl arylamine compound; The crude iron-based arylamine compound was washed with water, extracted with ethyl acetate, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com