Bipolar green light-based organic electroluminescent material and preparation method thereof
A luminescent, bipolar technology, applied in the field of bipolar green organic electroluminescent materials and their preparation, can solve the problem of low carrier transport efficiency, and achieve the ability to promote injection and transport, electron injection and transport The effect of enhancing and improving the effect of the device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 2-aminobenzaldehyde (4.8g, 40mmol) and 50mL of acetone into a 250mL round-bottomed flask, stir in an ice bath for 30min, then add 6% sodium hydroxide solution (120mL) dropwise to the system, and remove Ice bath, stirring at room temperature for 12 hours; then transfer the system to a 1000mL beaker, add an appropriate amount of ice water, 8% dilute hydrochloric acid solution to adjust the pH of the system to 5.5, a large number of yellow solids precipitated, suction filtered, and recrystallized with ethanol / water = 2:1 , to obtain compound (5.15, yield 80%) shown in formula (III), its chemical reaction equation is:
[0026]
Embodiment 2
[0028] To a 500 mL flask was added 4,7-dibromo-2,1,3-benzothiadiazole (2.95 g, 10 mmol), potassium carbonate (4 g, 30 mmol) and 80 mL of acetonitrile. After stirring and dissolving under argon, add the compound (4.8g.30mmol) shown in formula (III), stir and reflux at 85°C, TLC detects the reaction process; after the reaction is over, filter potassium carbonate, and remove the solvent under reduced pressure to obtain The crude product was purified by silica gel chromatography using petroleum / ethyl acetate=10 / 1 as eluent. Then the product is recrystallized in ethanol to obtain compound (6.54g, yield 72%) shown in formula (IV), and its chemical reaction equation is:
[0029]
Embodiment 3
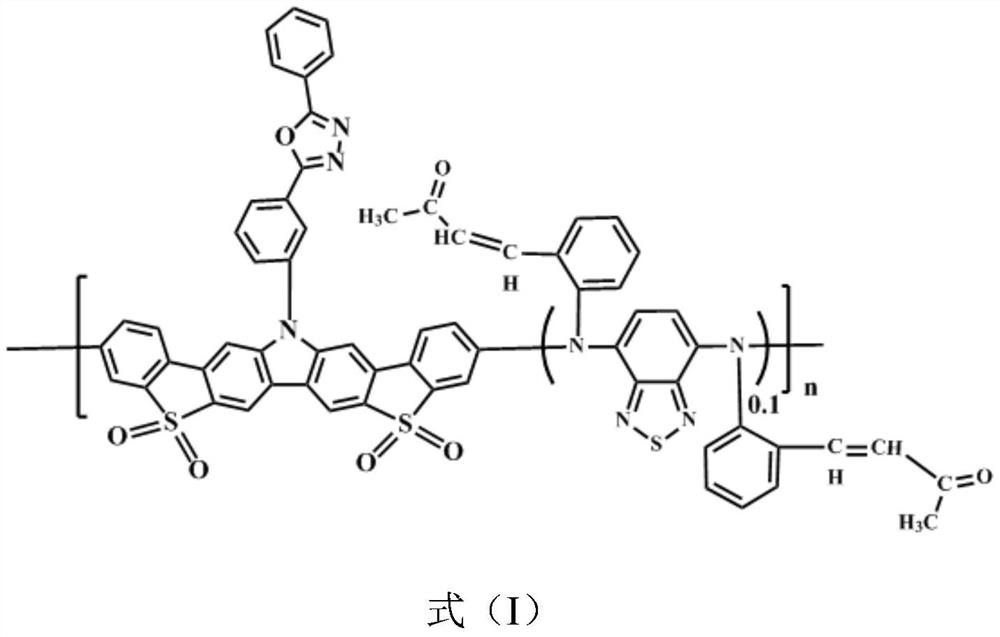
[0031] Add compound (4.54g, 10mmol) shown in formula (IV) in the single port reaction bottle of 250ml, then add chloroform solvent until raw material dissolves completely (100ml), NBS powder (7.12g, 40mmol) is dissolved in 30ml three Chloromethane solution was added dropwise into the reaction flask, and reacted in the dark for 24 hours. Extract three times with DCM, wash once with water, collect organic phase, spin dry DCM, then purify with THF recrystallization, obtain compound (11.3g, productive rate 92%) shown in formula (II), its chemical reaction equation is:
[0032]
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com