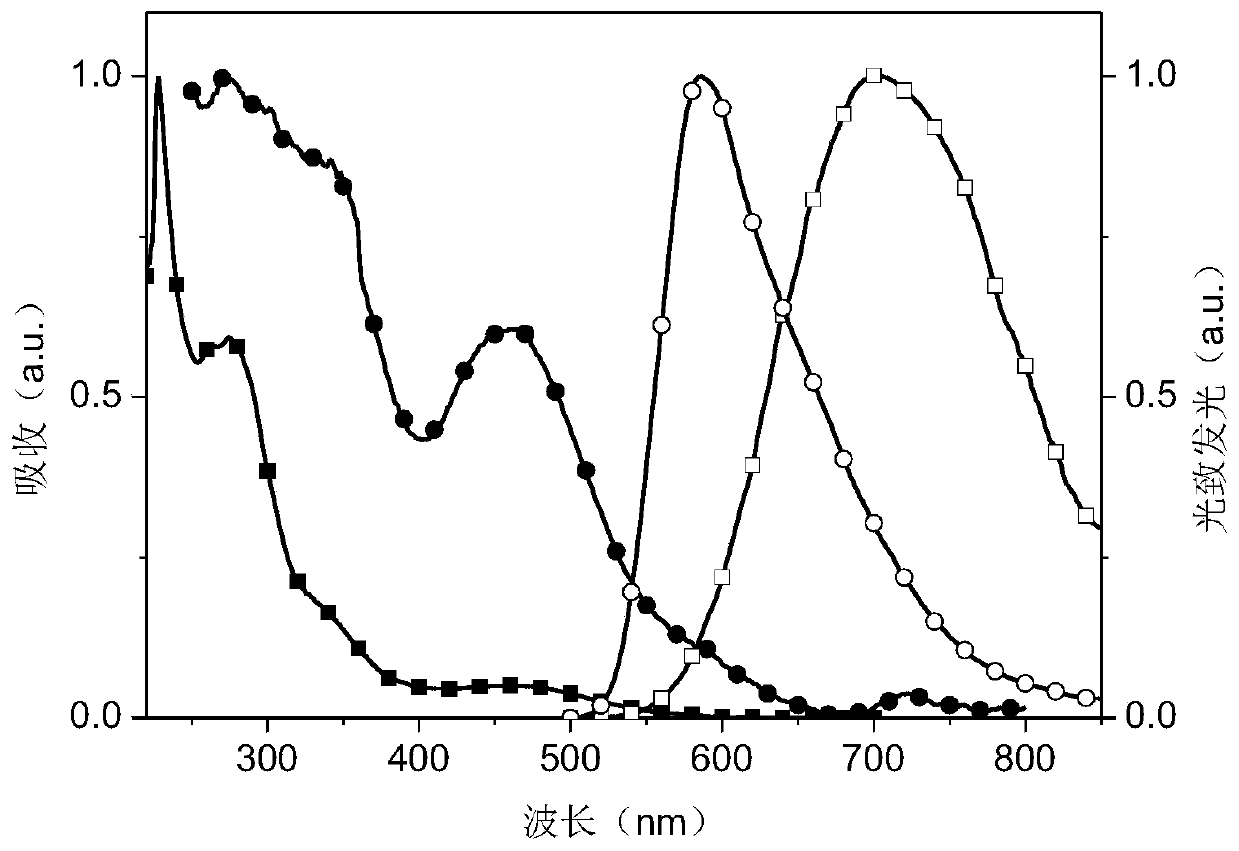
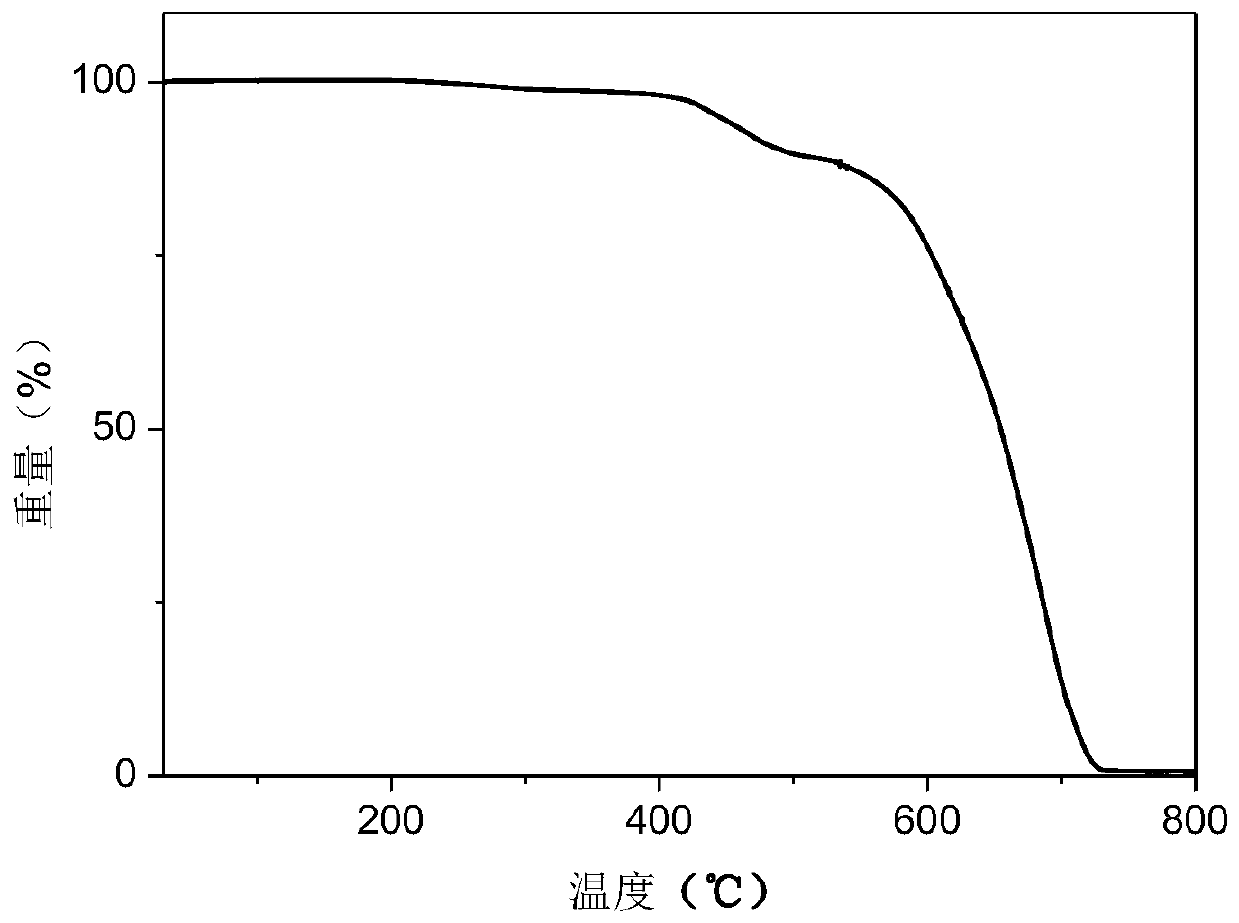
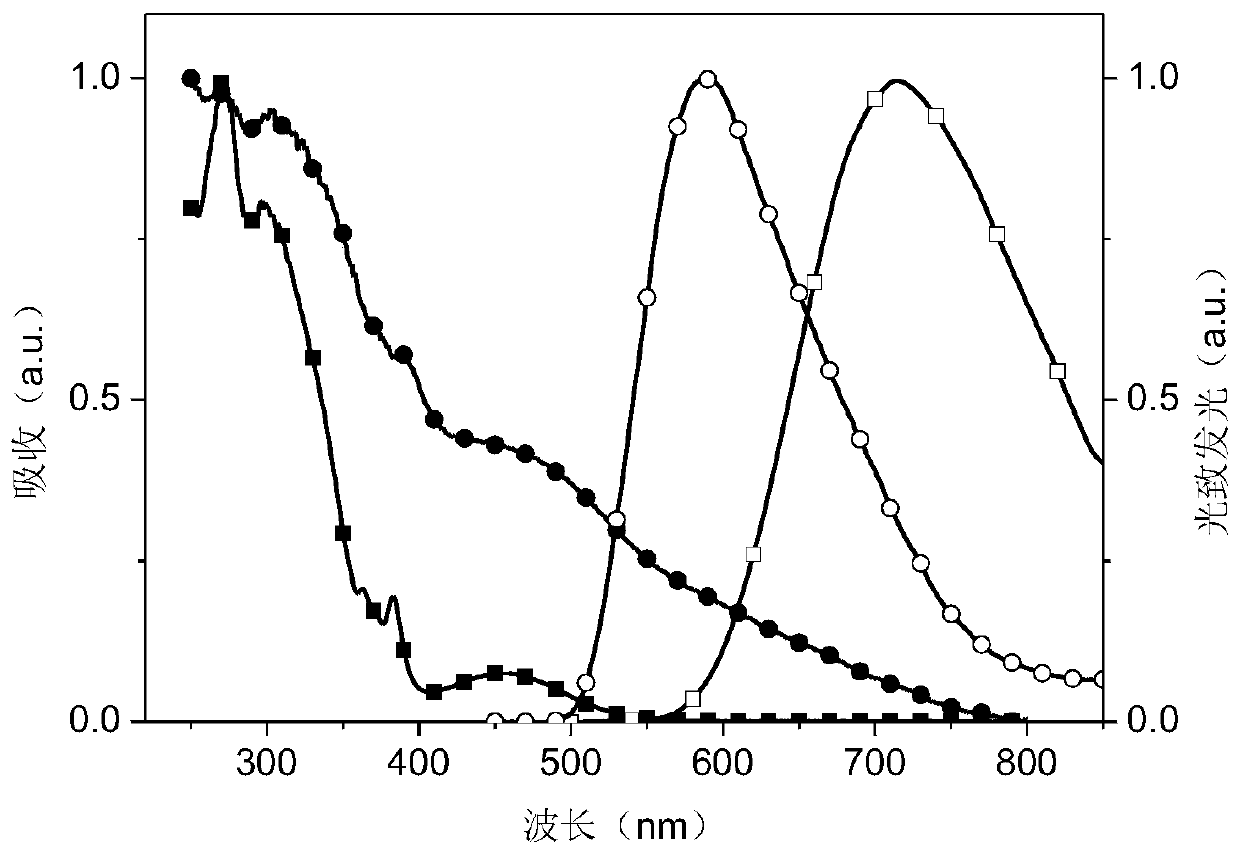
Dipyridine phenazinyl red/orange photothermal excitation delayed fluorescence material as well as synthesis method and application thereof
A technology of delayed fluorescence and synthetic methods, which is applied in the direction of luminescent materials, chemical instruments and methods, compounds of group 5/15 elements of the periodic table, etc., can solve problems such as concentration quenching, fast decay, and low efficiency of light-emitting devices, and achieve Improve efficiency, inhibit intermolecular interactions, and reduce quenching effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0058] Specific embodiment 1: In this embodiment, the structural formula of the dipyridophenazinyl red / orange photothermal excitation delayed fluorescence material is as follows:
[0059] where X is hydrogen or
[0060] Y is hydrogen,
[0061] Z is hydrogen,
[0062] W is hydrogen or
[0063] When X, Y, Z are hydrogen, W is , its structural formula is:
[0064]
[0065] When X, Y are hydrogen, W, Z are , its structural formula is:
[0066]
[0067] When W, Y, Z are hydrogen, X is , its structural formula is:
[0068]
[0069] When W and Z are hydrogen, X and Y are , its structural formula is:
[0070]
[0071] When X, Y are hydrogen, W is Z is , its structural formula is:
[0072]
[0073] When W and Z are hydrogen, X is Y is , its structural formula is:
[0074]
specific Embodiment approach 2
[0075] Specific embodiment 2: The synthesis method of the dipyridophenazinyl red / orange photothermal excitation delayed fluorescent material described in specific embodiment 1 is carried out according to the following steps:
[0076] 1. Mix 1 mmol of 5-bromobenzothiadiazole or 4-bromobenzothiadiazole, 1 mmol of triphenylamine-4-boronic acid pinacol ester, 0.05 to 0.1 mmol of tetrakis(triphenylphosphine) palladium, Mix 0.1mmol of tetrabutylammonium bromide, 2-10mmol of sodium hydroxide and 5-10ml of toluene, react at 120°C for 48h, extract with water and dichloromethane, combine the organic layers, remove the organic solvent after drying, and dichloromethane The mixed solvent with petroleum ether is eluent column chromatography and purified to obtain 4-(.benzo[c][1,2,5]thiadiazol-5-yl)-N,N-diphenylaniline or 4-(Benzo[c][1,2,5]thiadiazol-4-yl)-N,N-diphenylaniline;
[0077] 2. Add 1 mmol of 5,6-dibromobenzothiadiazole or 4,7-dibromobenzothiadiazole, 2 mmol of triphenylamine-4-bo...
specific Embodiment approach 3
[0082] Specific embodiment three: the difference between this embodiment and specific embodiment two is that in step one, 1 mmol of 5-bromobenzothiadiazole or 4-bromobenzothiadiazole, 1 mmol of triphenylamine-4-boric acid pina Alcohol ester, 0.05mmol of tetrakis(triphenylphosphine) palladium, 0.1mmol of tetrabutylammonium bromide, 6mmol of sodium hydroxide and 10ml of toluene were mixed and reacted at 120°C for 48h; in step 2, 1mmol of 5,6-di Bromobenzothiadiazole or 4,7-dibromobenzothiadiazole, 2mmol of triphenylamine-4-boronic acid pinacol ester, 0.05mmol of tetrakis(triphenylphosphine) palladium, 0.1mmol of tetrabutyl Ammonium bromide, 6mmol sodium hydroxide and 10ml toluene were mixed and reacted at 120°C for 48h. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com