Synthesis method of pomalidomide intermediate
A technology of pomalidomide and a synthesis method, which is applied in the field of chemical drug synthesis, can solve the problems of low product purity, high toxicity of raw materials, and many impurities, and achieves the effects of high product purity, simple and easy single-step reaction, and environmental protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] Solution preparation
[0044] Blank solution: take diluting solvent, that is.
Embodiment 1
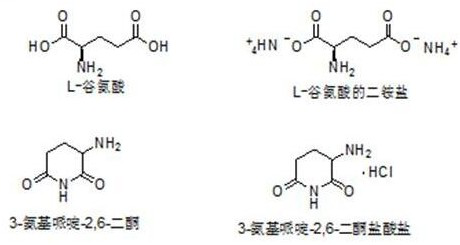
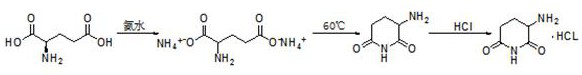
[0048] (1) Stir and disperse 29.4g of L-glutamic acid in 294g of water, slowly add 8.75g of ammonia water dropwise, keep the temperature below 15°C, and continue to heat and stir for 0.5h after dissolving to form the diammonium salt of L-glutamic acid.
[0049] (2) Heating to 60°C for 2 hours, the diammonium salt of L-glutamic acid removes a molecule of ammonia and cyclizes to form 3-aminopiperidine-2,6-dione, which is precipitated in water.
[0050] (3) Filter and dry at 105°C for 3 hours to obtain 21.4g of 3-aminopiperidine-2,6-dione. Dissolve 21g of 3-aminopiperidine-2,6-dione in 210g of ethanol and pour into the system Introduce hydrochloric acid gas, control the temperature to less than 15°C, until there is no more solid precipitation.
[0051] (4) Filter and dry at 70°C for 3 hours to obtain 27.0 g of the product 3-aminopiperidine-2,6-dione hydrochloride, with a total yield of 82.1% and a purity of 99.76%. (the product obtained in this embodiment uses the high-performan...
Embodiment 2
[0053] (1) Stir and disperse 29.4g of L-glutamic acid in 294g of water, slowly add 7g of ammonia water dropwise, keep the temperature below 15°C, and continue to heat and stir for 0.5h after dissolving to form the diammonium salt of L-glutamic acid.
[0054] (2) Heating to 60°C for 2 hours, the diammonium salt of L-glutamic acid removes a molecule of ammonia and cyclizes to form 3-aminopiperidine-2,6-dione, which is precipitated in water.
[0055] (3) Filter, dry at 105°C for 3 hours to obtain 18g of 3-aminopiperidine-2,6-dione, dissolve 18g of 3-aminopiperidine-2,6-dione in 180g of ethanol, and pass through the system Add hydrochloric acid gas and control the temperature to less than 15°C until no solid is precipitated.
[0056] (4) Filter and dry at 70°C for 3 hours to obtain 27.1 g of the product 3-aminopiperidine-2,6-dione hydrochloride, with a total yield of 82.3% and a purity of 99.35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com