Method for preparing methyl 2-phenyl-3-(2'-pyridyl)-3H-indole formate compound through aluminum catalysis
A technology for the catalytic preparation of methyl indole carboxylate, which is applied in the field of chemical intermediate preparation, and can solve the problems of many reaction steps, low reaction efficiency, and limited application range of the reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of 4a product
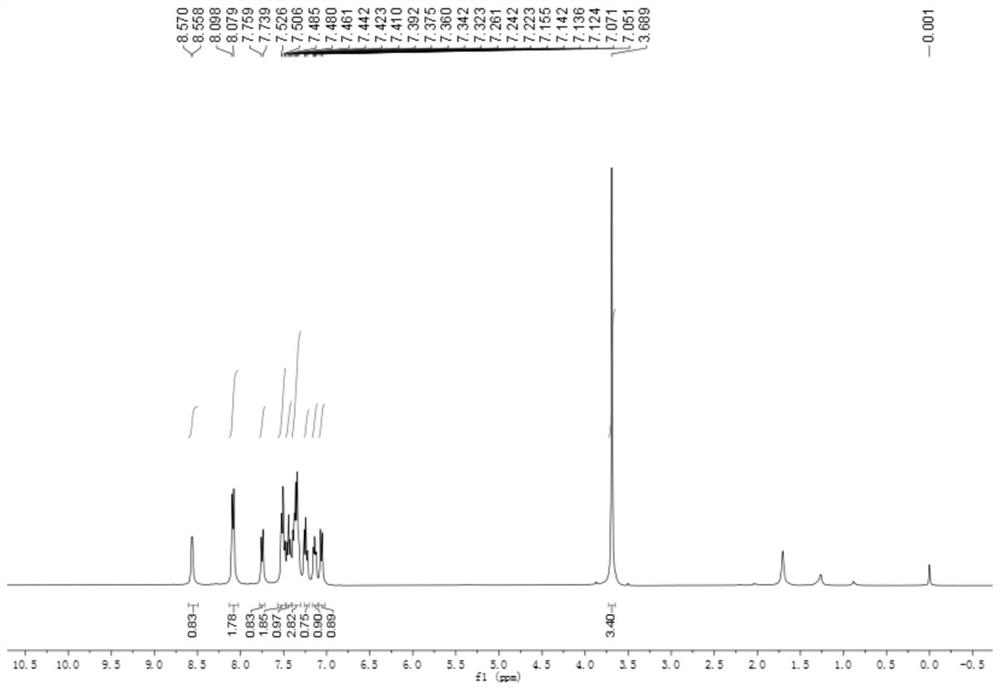
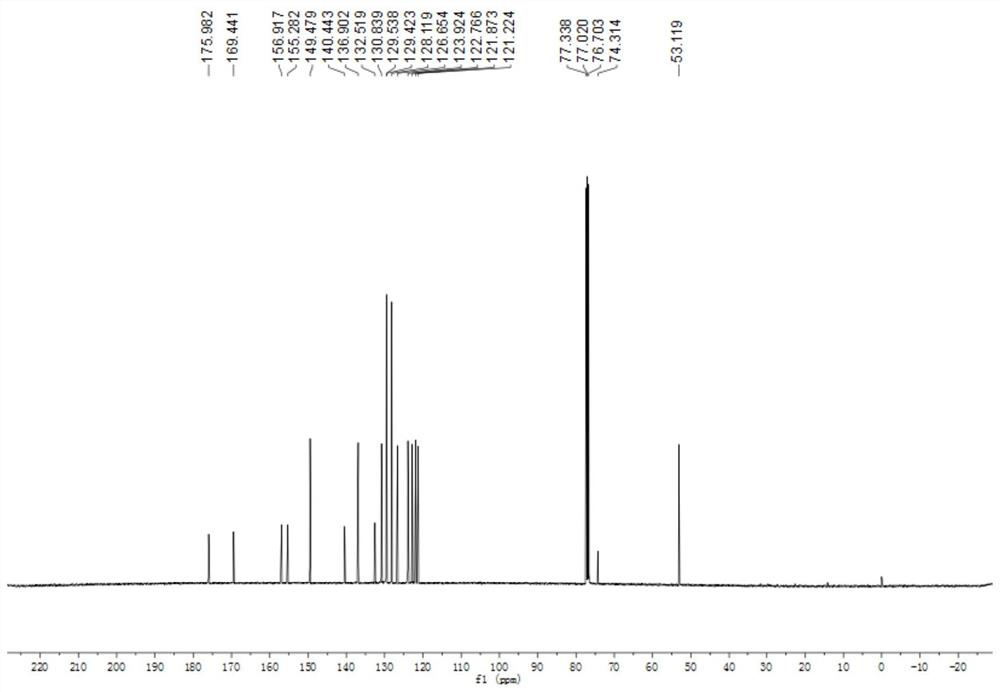
[0039] At room temperature, add 3mmol of 2-iodobenzoylformyl methyl ester, 6mmol of benzylamine and 3mmol of 2-pyridinecarbaldehyde into a 25mL round-bottom flask respectively, then add 10mL of DMF-DMSO mixed solution, 0.3 mmol of triethylaluminum and 6 mmol of sodium carbonate, the reaction was stirred at 70°C for 5 hours. After cooling, add 20mL saturated aqueous sodium chloride solution to the system, extract 3 times with ethyl acetate, 20mL each time, combine the organic phases, dry with anhydrous sodium sulfate, evaporate the solvent, and obtain by 200-300 mesh silica gel column chromatography Product 4a (90% yield, white solid). 4a 1 H-NMR spectrum see figure 1 , 4a 13 C-NMR spectrum see figure 2 .
[0040]
[0041] 2-Phenyl-3-(pyridin-2-yl)-3H-indole-3-carboxylate mp: 125.7-134.6℃; 1HNMR (400MHz, Chloroform-d) δ8.56(d, J=4.8Hz, 1H), 8.09(d, J=7.6Hz, 2H), 7.75(d, J=7.8Hz, 1H), 7.51(t, J=8.2Hz, 2H), 7.44(t, ...
Embodiment 2
[0044] Embodiment 2: Preparation of 4b product
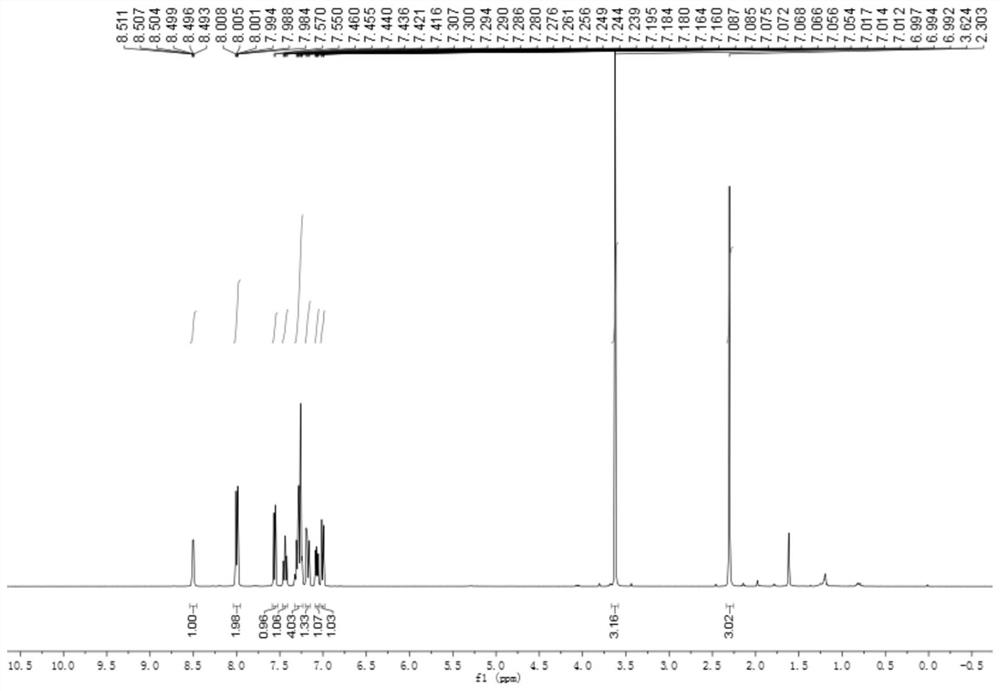
[0045] At room temperature, add 3mmol of 3-methyl-2-iodobenzoylformic acid methyl ester, 6mmol of benzylamine and 3mmol of 2-pyridinecarbaldehyde in a 25mL round bottom flask respectively, and then add 10mL of DMF- DMSO mixed solution, 0.3mmol of triethylaluminum and 6mmol of sodium carbonate were stirred at 70°C for 5 hours. After cooling, add 20 mL of saturated aqueous sodium chloride solution to the system, extract 3 times with ethyl acetate, 20 mL each time, combine the organic phases, dry with anhydrous sodium sulfate, evaporate the solvent, and obtain Product 4b (89% yield, white solid). 4b 1 H-NMR spectrum see image 3 , 4b 13 C-NMR spectrum see Figure 4 .
[0046]
[0047] 5-Methyl-2-phenyl-3-(pyridin-2-yl)-3H-indole-3-carboxylate mp: 172.7-175.7℃;
[0048] 1 H NMR (400MHz, Chloroform-d) δ8.50(dt, J=4.7,1.5Hz,1H),8.00(dt, J=6.9,1.6Hz,2H),7.56(d,J=7.8Hz,1H) ,7.44(td,J=7.8,1.9Hz,1H), 7.33–7.23(m,4H),7.21–7.15(...
Embodiment 3
[0051] Embodiment 3: Preparation of 4c product
[0052] At room temperature, add 3mmol of 3-methoxy-2-iodobenzoylformic acid methyl ester, 6mmol of benzylamine and 3mmol of 2-pyridinecarbaldehyde in a 25mL round bottom flask respectively, and then add 10mL of DMF in sequence - DMSO mixed solution, 0.3 mmol of triethylaluminum and 6 mmol of sodium carbonate, and the reaction was stirred at 70° C. for 5 hours. After cooling, add 20 mL of saturated aqueous sodium chloride solution to the system, extract 3 times with ethyl acetate, 20 mL each time, combine the organic phases, dry with anhydrous sodium sulfate, evaporate the solvent, and obtain Product 4c (86% yield, white solid). 4c 1 H-NMR spectrum see Figure 5 , 4c 13 C-NMR spectrum see Figure 6 .
[0053]
[0054] 5-Methoxy-2-phenyl-3-(pyridin-2-yl)-3H-indole-3-carboxylate mp: 162.7-165.4℃;
[0055] 1 H NMR (400MHz, Chloroform-d) δ8.59(dt, J=4.5, 1.5Hz, 1H), 8.09–8.01(m, 2H), 7.64(d, J=8.5Hz, 1H), 7.49(td, J=7.8,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com