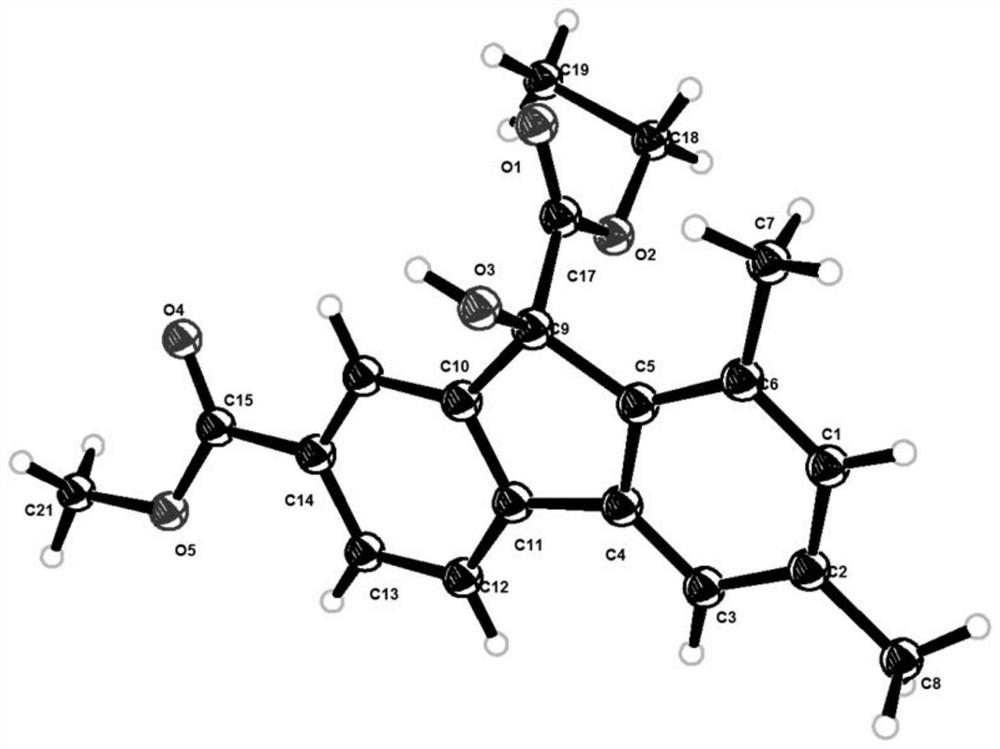
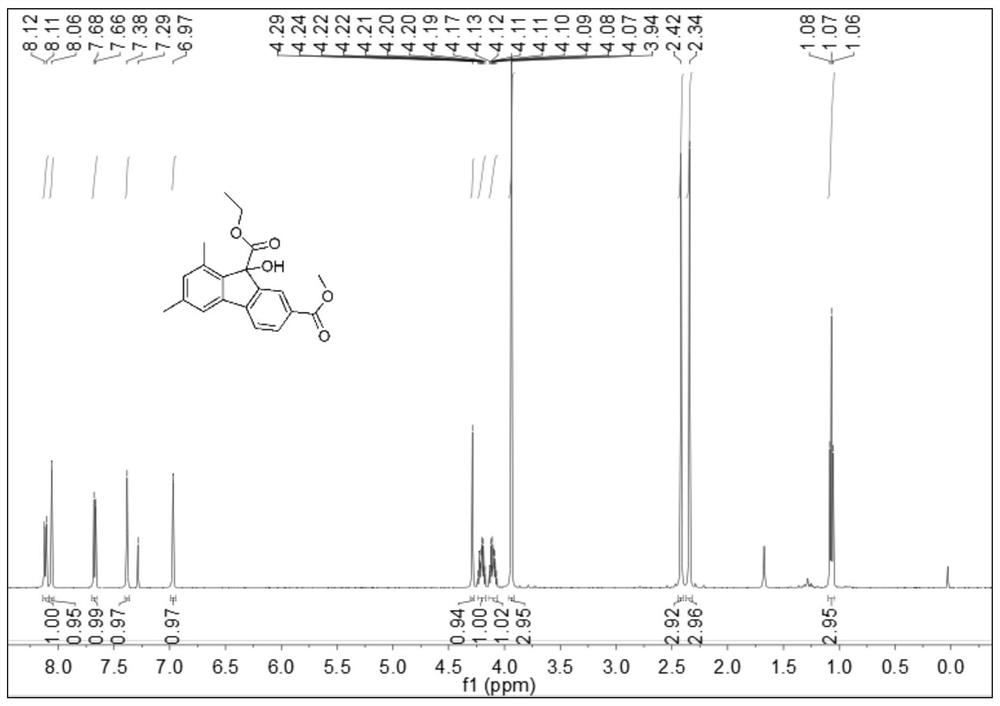
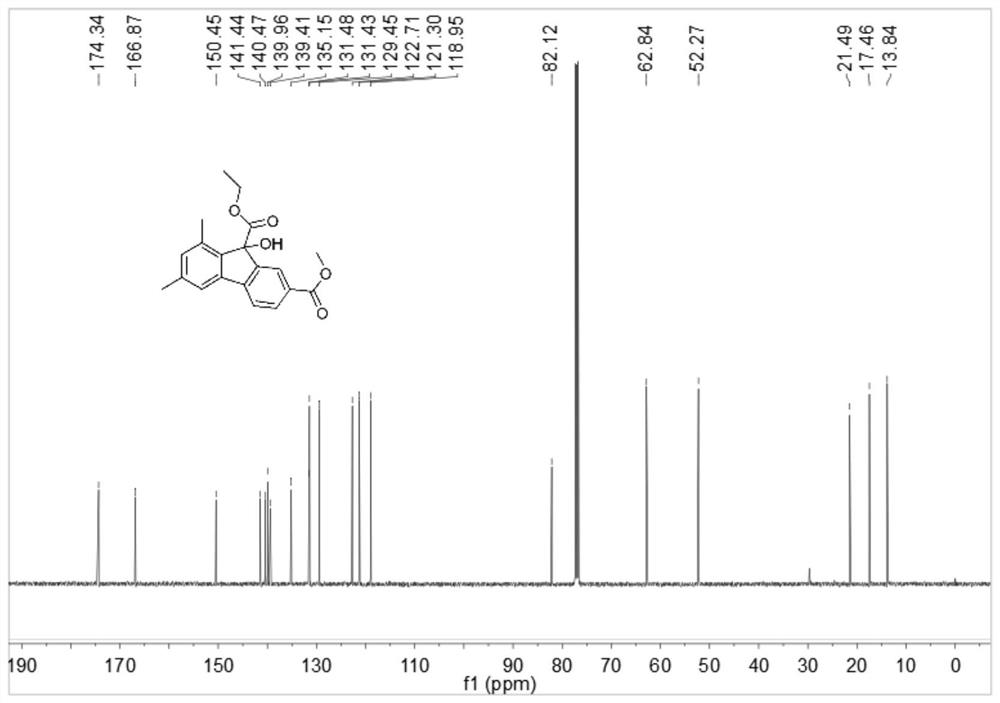
Simple preparation method of 9-hydroxyfluorene-9-carboxylate compound
A technology of ester compound and hydroxyfluorene, applied in the field of organic synthesis, can solve problems such as difficulty in obtaining raw materials, and achieve the effects of less reaction by-products, alleviating the pressure on the environment, and less waste discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of 9-ethyl-2-methyl-9-hydroxy-6,8-dimethyl-9H-fluorene-2,9-dicarboxylate:
[0040] Preparation of 9-ethyl-2-methyl-9-hydroxy-6,8 from ethyl 2-(2,4-dimethylphenyl)-2-oxoacetate and methyl 4-iodobenzoate -Dimethyl-9H-fluorene-2,9-dicarboxylate, the chemical reaction formula is as follows:
[0041]
[0042] Specific steps are as follows:
[0043] Add palladium acetate (Pd(OAc) 2 ) 4.49mg (0.02mmol), ethyl 2-(2,4-dimethylphenyl)-2-oxoacetate 41.2mg (0.2mmol), methyl 4-iodobenzoate 131.0mg (0.5mmol), Silver trifluoroacetate (AgTFA) 88.4mg (0.4mmol), trifluoroacetic acid (TFA) 136.8mg (1.2mmol) and 2-fluoro-5-trifluoromethylaniline 14.3mg (0.08mmol), 1.8mL hexafluoroiso Propanol, 0.2mL anhydrous acetic acid, put the reaction solution in a heater at 100°C and stir for 48h, the passing specification is The thin-layer chromatographic plate of F-254 is tracked, and the reaction raw material is no longer consumed and reduced, and the product is no longer increas...
Embodiment 2
[0046] Preparation of 9-hydroxyfluorene-9-carboxylate compound as 9-hydroxy-6,8-dimethyl-9H-fluorene-2,9-dicarboxylate diethyl ester:
[0047] Preparation of 9-hydroxy-6,8-dimethyl-9H-fluorene-2 from ethyl 2-(2,4-dimethylphenyl)-2-oxoacetate and ethyl 4-iodobenzoate , 9- diethyl carboxylate, the chemical reaction formula is as follows:
[0048]
[0049] Specific steps are as follows:
[0050] Add 4.49mg (0.02mmol) of palladium acetate, 41.2mg (0.2mmol) of formyl ethyl 2,4-dimethylbenzoate, and 138.0mg (0.5mmol) of ethyl 4-iodobenzoate into a sealed pressure-resistant tube. ), silver trifluoroacetate (AgTFA) 88.4mg (0.4mmol), trifluoroacetic acid 136.8mg (1.2mmol) and 2-fluoro-5-trifluoromethylaniline 14.3mg (0.08mmol), 1.8mL hexafluoroisopropyl Alcohol, 0.2mL of anhydrous acetic acid, put the reaction solution in a heater at 100°C and stir for 48 hours, the passing specification is The thin-layer chromatographic plate of F-254 is tracked, and the reaction raw material...
Embodiment 3
[0053] Preparation of 9-hydroxyfluorene-9-carboxylate compound as ethyl 9-hydroxy-1,3-dimethyl-5-nitro-9H-fluorene-9-carboxylate:
[0054] Preparation of 9-hydroxy-1,3-dimethyl-5-nitro-9H- from 2-(2,4-dimethylphenyl)-2-oxoethyl acetate and o-nitroiodobenzene Ethyl fluorene-9-carboxylate, the chemical reaction formula is as follows:
[0055]
[0056] Specific steps are as follows:
[0057] Add 4.49 mg (0.02 mmol) of palladium acetate, 41.2 mg (0.2 mmol) of ethyl 2-(2,4-dimethylphenyl)-2-oxoacetate, and 124.5 mg of o-nitroiodobenzene into a sealed pressure-resistant tube. mg (0.5mmol), silver trifluoroacetate (AgTFA) 88.4mg (0.4mmol), trifluoroacetic acid 136.8mg (1.2mmol) and 2-fluoro-5-trifluoromethylaniline 14.3mg (0.08mmol), 1.8mL Hexafluoroisopropanol, 0.2mL of anhydrous acetic acid, put the reaction solution in a heater at 100°C and stir for 48 hours, the passing specification is The thin-layer chromatographic plate of F-254 is tracked, and the reaction raw material...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com